Cone outer segment extracellular matrix as binding domain for interphotoreceptor retinoid-binding protein
- PMID: 21935947
- PMCID: PMC4128833
- DOI: 10.1002/cne.22773
Cone outer segment extracellular matrix as binding domain for interphotoreceptor retinoid-binding protein
Abstract
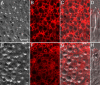
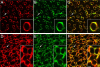
Cones are critically dependent on interphotoreceptor retinoid-binding protein (IRBP) for retinoid delivery in the visual cycle. Cone-dominant vertebrates offer an opportunity to uncover the molecular basis of IRBP's role in this process. Here, we explore the association of IRBP with the interphotoreceptor matrix (IPM) of cones vs. rods in cone dominant retinas from chicken (Gallus domesticus), turkey (Meleagris gallopavo), and pig (Sus scrofa). Retinas were detached and fixed directly or washed in saline prior to fixation. Disassociated photoreceptors with adherent matrix were also prepared. Under 2 mM CaCl(2) , insoluble matrix was delaminated from saline washed retinas. The distribution of IRBP, as well as glycans binding peanut agglutinin (cone matrix) and wheat germ agglutinin (rod/cone matrix), was defined by confocal microscopy. Retina flat mounts showed IRBP diffusely distributed in an interconnecting, lattice-like pattern throughout the entire matrix. Saline wash replaced this pattern with fluorescent annuli surrounding individual cone outer segments. In isolated cones and matrix sheets, IRBP colocalized with the peanut agglutinin binding matrix glycans. Our results reveal a wash-resistant association of IRBP with a matrix domain immediately surrounding cone outer segments. The cone matrix sheath may be responsible for IRBP-mediated cone targeting of 11-cis retinoids.
Copyright © 2011 Wiley Periodicals, Inc.
Figures







References
-
- Adler AJ, Evans CD. Rapid isolation of bovine interphotoreceptor retinol-binding protein. Biochim Biophys Acta. 1983;761:217–222. - PubMed
-
- Adler AJ, Chader GJ, Wiggert B. Purification and assay of interphotoreceptor retinoid-binding protein from the eye. Methods Enzymol. 1990;189:213–223. - PubMed
-
- Anderson DH, Neitz J, Saari JC, Kaska DD, Fenwick J, Jacobs GH, Fisher SK. Retinoid-binding proteins in cone-dominant retinas. Invest Ophthalmol Vis Sci. 1986;27:1015–1026. - PubMed
-
- Blanks JC, Johnson LV. Specific binding of peanut lectin to a class of retinal photoreceptor cells. A species comparison. Invest Ophthalmol Vis Sci. 1984;25:546–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

