Cytoprotective and proangiogenic activity of ex-vivo netrin-1 transgene overexpression protects the heart against ischemia/reperfusion injury
- PMID: 21936706
- PMCID: PMC3376469
- DOI: 10.1089/scd.2011.0475
Cytoprotective and proangiogenic activity of ex-vivo netrin-1 transgene overexpression protects the heart against ischemia/reperfusion injury
Abstract
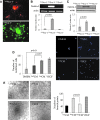
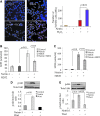
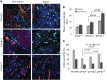
In continuation of a previous work that transgene expression of sonic hedgehog promoted neo-vascularization via netrin-1 release, the current study was aimed at assessing the anti-apoptotic and pro-angiogenic role of netrin-1 transgene overexpression in the ischemic myocardium. pLP-Adeno-X ViralTrak vectors containing netrin-1 cDNA amplified from rat mesenchymal stem cells (Ad-netrin) or without a therapeutic gene (Ad-null) were constructed and transfected into HEK-293 cells to produce Ad-netrin and Ad-null vectors. Sca-1(+)-like cells were isolated and propagated in vitro and were successfully transduced with Ad-netrin transduced Sca-1(+) cells ((Net)Sca-1(+)) and Ad-null transduced Sca-1(+) cells ((Null)Sca-1(+)). Overexpression of netrin-1 in (Net)Sca-1(+) was confirmed by reverse transcription-polymerase chain reaction and western blot. Neonatal cardiomyocytes and rat endothelial cells expressed netrin-1 specific receptor Uncoordinated-5b and the conditioned medium from (Net)Sca-1(+) cells was protective for both the cell types against oxidant stress. For in vivo studies, the rat model of myocardial ischemia/reperfusion injury was developed in female Wistar rats by left anterior descending coronary artery occlusion for 45 min followed by reperfusion. The animals were grouped to receive 70 μL of Dulbecco's modified Eagle's medium without cells (group-1), containing 2×10(6) (Null)Sca-1(+) cells (group-2) and (Net)Sca-1(+) cells (group-3). (Net)Sca-1(+) cells significantly reduced ischemia/reperfusion injury in the heart and preserved the global heart function in group-3 (P<0.05 vs. groups-1 and group-2). Ex-vivo netrin-1 overexpression in the heart increased NOS activity in the heart. Blood vessel density was significantly higher in group-3 (P<0.05 vs. controls). We concluded that netrin-1 decreased apoptosis in cardiomyocytes and endothelial cells via activation of Akt. Netrin-1 transgene expression was proangiogenic and effectively reduced ischemia/reperfusion injury to preserve global heart function.
Figures


Similar articles
-
Activation of diverse signaling pathways by ex-vivo delivery of multiple cytokines for myocardial repair.Stem Cells Dev. 2013 Jan 15;22(2):204-15. doi: 10.1089/scd.2011.0575. Epub 2012 Oct 5. Stem Cells Dev. 2013. PMID: 22873203 Free PMC article.
-
Induction of cardioprotection by small netrin-1-derived peptides.Am J Physiol Cell Physiol. 2015 Jul 15;309(2):C100-6. doi: 10.1152/ajpcell.00332.2014. Epub 2015 Apr 29. Am J Physiol Cell Physiol. 2015. PMID: 25924621 Free PMC article.
-
AAV-mediated netrin-1 overexpression increases peri-infarct blood vessel density and improves motor function recovery after experimental stroke.Neurobiol Dis. 2011 Oct;44(1):73-83. doi: 10.1016/j.nbd.2011.06.006. Epub 2011 Jun 25. Neurobiol Dis. 2011. PMID: 21726647 Free PMC article.
-
Shenxian-Shengmai Oral Liquid Reduces Myocardial Oxidative Stress and Protects Myocardium from Ischemia-Reperfusion Injury.Cell Physiol Biochem. 2018;48(6):2503-2516. doi: 10.1159/000492688. Epub 2018 Aug 17. Cell Physiol Biochem. 2018. PMID: 30121659
-
Mechanisms of endothelial cell migration.Cell Mol Life Sci. 2014 Nov;71(21):4131-48. doi: 10.1007/s00018-014-1678-0. Epub 2014 Jul 20. Cell Mol Life Sci. 2014. PMID: 25038776 Free PMC article. Review.
Cited by
-
Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue.Acta Biomater. 2018 Mar 15;69:95-106. doi: 10.1016/j.actbio.2017.12.025. Epub 2017 Dec 24. Acta Biomater. 2018. PMID: 29281806 Free PMC article.
-
Netrin-1 mediates nerve innervation and angiogenesis leading to discogenic pain.J Orthop Translat. 2022 Dec 23;39:21-33. doi: 10.1016/j.jot.2022.11.006. eCollection 2023 Mar. J Orthop Translat. 2022. PMID: 36605621 Free PMC article.
-
Netrin 1 regulates blood-brain barrier function and neuroinflammation.Brain. 2015 Jun;138(Pt 6):1598-612. doi: 10.1093/brain/awv092. Epub 2015 Apr 22. Brain. 2015. PMID: 25903786 Free PMC article.
-
The road ahead: working towards effective clinical translation of myocardial gene therapies.Ther Deliv. 2014 Jan;5(1):39-51. doi: 10.4155/tde.13.134. Ther Deliv. 2014. PMID: 24341816 Free PMC article. Review.
-
Netrin-1 attenuates cardiac ischemia reperfusion injury and generates alternatively activated macrophages.Inflammation. 2014 Apr;37(2):573-80. doi: 10.1007/s10753-013-9771-3. Inflammation. 2014. PMID: 24234226
References
-
- Baioni L. Basini G. Bussolati S. Cortimiglia C. Grasselli F. Netrin-1: just an axon-guidance factor? Vet Res Commun. 2010;34(Suppl 1):S1–S4. - PubMed
-
- Li Q. Yao D. Ma J. Zhu J. Xu X. Ren Y. Ding X. Mao X. Transplantation of MSCs in Combination with Netrin-1 Improves Neoangiogenesis in a Rat Model of Hind Limb Ischemia. J Surg Res. 2009;166:162–169. - PubMed
-
- Castets M. Mehlen P. Netrin-1 role in angiogenesis: to be or not to be a pro-angiogenic factor? Cell Cycle. 2010;9:1466–1471. - PubMed
-
- Castets M. Coissieux MM. Delloye-Bourgeois C. Bernard L. Delcros JG. Bernet A. Laudet V. Mehlen P. Inhibition of endothelial cell apoptosis by netrin-1 during angiogenesis. Dev Cell. 2009;16:614–620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

