Myosin motor proteins are involved in the final stages of the secretory pathways
- PMID: 21936774
- PMCID: PMC3403808
- DOI: 10.1042/BST0391115
Myosin motor proteins are involved in the final stages of the secretory pathways
Abstract
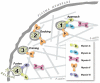
In eukaryotes, the final steps in both the regulated and constitutive secretory pathways can be divided into four distinct stages: (i) the 'approach' of secretory vesicles/granules to the PM (plasma membrane), (ii) the 'docking' of these vesicles/granules at the membrane itself, (iii) the 'priming' of the secretory vesicles/granules for the fusion process, and, finally, (iv) the 'fusion' of vesicular/granular membranes with the PM to permit content release from the cell. Recent work indicates that non-muscle myosin II and the unconventional myosin motor proteins in classes 1c/1e, Va and VI are specifically involved in these final stages of secretion. In the present review, we examine the roles of these myosins in these stages of the secretory pathway and the implications of their roles for an enhanced understanding of secretion in general.
Figures
References
-
- Kelly RB. Pathways of protein secretion in eukaryotes. Science. 1985;230:25–32. - PubMed
-
- Burgess TL, Kelly RB. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. - PubMed
-
- Griffiths G, Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986;234:438–443. - PubMed
-
- Saraste J, Svensson K. Distribution of the intermediate elements operating in ER to Golgi transport. J Cell Sci. 1991;100(Pt 3):415–430. - PubMed
-
- Sollner TH. Regulated exocytosis and SNARE function (Review) Mol Membr Biol. 2003;20:209–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

