Targeting adenosine monophosphate-activated protein kinase (AMPK) in preclinical models reveals a potential mechanism for the treatment of neuropathic pain
- PMID: 21936900
- PMCID: PMC3186752
- DOI: 10.1186/1744-8069-7-70
Targeting adenosine monophosphate-activated protein kinase (AMPK) in preclinical models reveals a potential mechanism for the treatment of neuropathic pain
Abstract
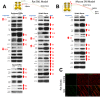
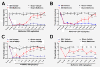
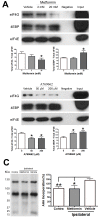
Neuropathic pain is a debilitating clinical condition with few efficacious treatments, warranting development of novel therapeutics. We hypothesized that dysregulated translation regulation pathways may underlie neuropathic pain. Peripheral nerve injury induced reorganization of translation machinery in the peripheral nervous system of rats and mice, including enhanced mTOR and ERK activity, increased phosphorylation of mTOR and ERK downstream targets, augmented eIF4F complex formation and enhanced nascent protein synthesis. The AMP activated protein kinase (AMPK) activators, metformin and A769662, inhibited translation regulation signaling pathways, eIF4F complex formation, nascent protein synthesis in injured nerves and sodium channel-dependent excitability of sensory neurons resulting in a resolution of neuropathic allodynia. Therefore, injury-induced dysregulation of translation control underlies pathology leading to neuropathic pain and reveals AMPK as a novel therapeutic target for the potential treatment of neuropathic pain.
Figures
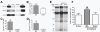


Similar articles
-
Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain.Mol Pain. 2012 Jan 23;8:5. doi: 10.1186/1744-8069-8-5. Mol Pain. 2012. PMID: 22269797 Free PMC article.
-
AMPK activation by peri-sciatic nerve administration of ozone attenuates CCI-induced neuropathic pain in rats.J Mol Cell Biol. 2017 Apr 1;9(2):132-143. doi: 10.1093/jmcb/mjw043. J Mol Cell Biol. 2017. PMID: 27744376
-
Proteomic and functional annotation analysis of injured peripheral nerves reveals ApoE as a protein upregulated by injury that is modulated by metformin treatment.Mol Pain. 2013 Mar 26;9:14. doi: 10.1186/1744-8069-9-14. Mol Pain. 2013. PMID: 23531341 Free PMC article.
-
Targeting AMPK for the Alleviation of Pathological Pain.Exp Suppl. 2016;107:257-285. doi: 10.1007/978-3-319-43589-3_11. Exp Suppl. 2016. PMID: 27812984 Free PMC article. Review.
-
AMPK: An emerging target for modification of injury-induced pain plasticity.Neurosci Lett. 2013 Dec 17;557 Pt A(0 0):9-18. doi: 10.1016/j.neulet.2013.06.060. Epub 2013 Jul 3. Neurosci Lett. 2013. PMID: 23831352 Free PMC article. Review.
Cited by
-
Glutamate Stimulates Local Protein Synthesis in the Axons of Rat Cortical Neurons by Activating α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors and Metabotropic Glutamate Receptors.J Biol Chem. 2015 Aug 21;290(34):20748-20760. doi: 10.1074/jbc.M115.638023. Epub 2015 Jul 1. J Biol Chem. 2015. PMID: 26134564 Free PMC article.
-
eIF2α phosphorylation controls thermal nociception.Proc Natl Acad Sci U S A. 2016 Oct 18;113(42):11949-11954. doi: 10.1073/pnas.1614047113. Epub 2016 Oct 3. Proc Natl Acad Sci U S A. 2016. PMID: 27698114 Free PMC article.
-
Activation of mammalian target of rapamycin contributes to pain nociception induced in rats by BmK I, a sodium channel-specific modulator.Neurosci Bull. 2014 Feb;30(1):21-32. doi: 10.1007/s12264-013-1377-0. Epub 2013 Oct 16. Neurosci Bull. 2014. PMID: 24132796 Free PMC article.
-
Chemotherapy-Induced Peripheral Neuropathy: Mechanisms and Therapeutic Avenues.Neurotherapeutics. 2021 Oct;18(4):2384-2396. doi: 10.1007/s13311-021-01142-2. Epub 2021 Oct 21. Neurotherapeutics. 2021. PMID: 34676514 Free PMC article. Review.
-
Metformin: A Prospective Alternative for the Treatment of Chronic Pain.Front Pharmacol. 2020 Sep 23;11:558474. doi: 10.3389/fphar.2020.558474. eCollection 2020. Front Pharmacol. 2020. PMID: 33178015 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

