The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial
- PMID: 21937481
- PMCID: PMC3383100
- DOI: 10.1093/jac/dkr375
The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial
Abstract
Objectives: Host iron availability is fundamental to mucormycosis pathogenesis. The combination of liposomal amphotericin B (LAmB) and deferasirox iron chelation therapy synergistically improved survival in diabetic mice with mucormycosis. To determine the safety of combination deferasirox plus LAmB therapy for mucormycosis, a multicentred, placebo-controlled, double-blinded clinical trial was conducted.
Methods: Twenty patients with proven or probable mucormycosis were randomized to receive treatment with LAmB plus deferasirox (20 mg/kg/day for 14 days) or LAmB plus placebo (NCT00419770, clinicaltrials.gov). The primary analyses were for safety and exploratory efficacy.
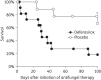
Results: Patients in the deferasirox arm (n=11) were more likely than those in the placebo arm (n=9) to have active malignancy, neutropenia and corticosteroid therapy, and were less likely to receive concurrent non-study antifungal therapy. Reported adverse events and serious adverse events were similar between the groups. However, death was more frequent in the deferasirox than in the placebo arm at 30 days (45% versus 11%, P=0.1) and 90 days (82% versus 22%, P=0.01). Global success (alive, clinically stable, radiographically improved) for the deferasirox arm versus the placebo arm at 30 and 90 days, respectively, was 18% (2/11) versus 67% (6/9) (P=0.06) and 18% (2/11) versus 56% (5/9) (P=0.2).
Conclusions: Patients with mucormycosis treated with deferasirox had a higher mortality rate at 90 days. Population imbalances in this small Phase II study make generalizable conclusions difficult. Nevertheless, these data do not support a role for initial, adjunctive deferasirox therapy for mucormycosis.
Figures
Comment in
-
Deferasirox in mucormycosis: hopefully, not defeated.J Antimicrob Chemother. 2012 Mar;67(3):783-4. doi: 10.1093/jac/dkr529. Epub 2011 Dec 13. J Antimicrob Chemother. 2012. PMID: 22167245 No abstract available.
-
Deferasirox as adjunctive therapy for mucormycosis.J Antimicrob Chemother. 2012 Mar;67(3):519-20. doi: 10.1093/jac/dkr540. Epub 2011 Dec 20. J Antimicrob Chemother. 2012. PMID: 22186877
References
-
- Ibrahim AS, Gebermariam T, Fu Y, et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117:2649–57. doi:10.1172/JCI32338. - DOI - PMC - PubMed
-
- Liu M, Spellberg B, Phan QT, et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 2010;120:1914–24. doi:10.1172/JCI42164. - DOI - PMC - PubMed
-
- Abe F, Shibuya H, Tateyama M, et al. Mucormycosis in diabetic ketoacidosis. Role of unbound iron binding capacity of transferrin. Acta Pathol Jpn. 1986;36:1507–12. - PubMed
-
- Artis WM, Fountain JA, Delcher HK, et al. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability. Diabetes. 1982;31:1109–14. doi:10.2337/diabetes.31.12.1109. - DOI - PubMed
-
- Eiser AR, Slifkin RF, Neff MS. Intestinal mucormycosis in hemodialysis patients following deferoxamine. Am J Kidney Dis. 1987;10:71–3. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical