A randomized, double-blind, active-controlled, double-dummy, parallel-group study to determine the safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate/severe, chronic cancer pain
- PMID: 21937568
- PMCID: PMC3255516
- DOI: 10.1177/0269216311418869
A randomized, double-blind, active-controlled, double-dummy, parallel-group study to determine the safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate/severe, chronic cancer pain
Abstract
Objective: An examination of whether oxycodone/naloxone prolonged-release tablets (OXN PR) can improve constipation and maintain analgesia, compared with oxycodone prolonged-release tablets (OxyPR) in patients with moderate/severe cancer pain.
Methods: Randomized, double-blind, active-controlled, double-dummy, parallel-group study in which 185 patients were randomized to receive up to 120 mg/day of OXN PR or OxyPR over 4 weeks. Efficacy assessments included Bowel Function Index (BFI), Brief Pain Inventory Short-Form (BPI-SF), laxative and rescue medication use. Quality of life (QoL) and safety assessments were conducted.
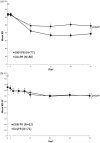
Results: After 4 weeks, mean BFI score was significantly lower with OXN PR; mean total laxative intake was 20% lower with OXN PR. Mean BPI-SF scores were similar for both treatments and the average rate of analgesic rescue medication use was low and comparable. QoL assessments were stable and comparable with greater improvements in constipation-specific QoL assessments with OXN PR. Overall, rates of adverse drug reactions were similar.
Conclusions: OXN PR provides superior bowel function in cancer pain patients, compared with OxyPR, without compromising analgesic efficacy or safety. This study confirms that OXN PR is well tolerated and efficacious in cancer pain patients and results are in line with those seen in non-malignant pain patients.
Trial registration: ClinicalTrials.gov NCT00513656.
Figures

References
-
- WHO Expert Committee Cancer pain relief and palliative care. Geneva: World Health Organization, 1990 - PubMed
-
- Jost L, Roila F. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol 2010; 21(Suppl. 5): v257–v260 - PubMed
-
- Scottish Intercollegiate Guidelines Network. 106 Control of pain in adults with cancer: A national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network, 2008
-
- NCCN NCCN clinical practice guidelines in oncology™: adult cancer pain – V.1.2010, http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed November 2010).
-
- Riley J, Eisenberg E, Müller-Schwefe G, et al. Oxycodone: a review of its use in the management of pain. Curr Med Res Opin 2008; 24: 175–192 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

