Conversion of lipid transfer inhibitor protein (apolipoprotein F) to its active form depends on LDL composition
- PMID: 21937674
- PMCID: PMC3220293
- DOI: 10.1194/jlr.M018283
Conversion of lipid transfer inhibitor protein (apolipoprotein F) to its active form depends on LDL composition
Abstract
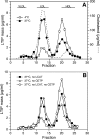
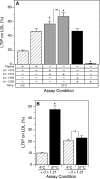
Lipid transfer inhibitor protein (LTIP) exists in both active and inactive forms. Incubation (37°C) of plasma causes LTIP to transfer from a 470 kDa inactive complex to LDL where it is active. Here, we investigate the mechanisms underlying this movement. Inhibiting LCAT or cholesteryl ester transfer protein (CETP) reduced incubation-induced LTIP translocation by 40-50%. Blocking both LCAT and CETP completely prevented LTIP movement. Under appropriate conditions, either factor alone could drive maximum LTIP transfer to LDL. These data suggest that chemical modification of LDL, the 470 kDa complex, or both facilitate LTIP movement. To test this, LDL and the 470 kDa fraction were separately premodified by CETP and/or LCAT activity. Modification of the 470 kDa fraction had no effect on subsequent LTIP movement to native LDL. Premodification of LDL, however, induced spontaneous LTIP movement from the native 470 kDa particle to LDL. This transfer depended on the extent of LDL modification and correlated negatively with changes in the LDL phospholipid + cholesterol-to-cholesteryl ester + triglyceride ratio. We conclude that LTIP translocation is dependent on LDL lipid composition, not on its release from the inactive complex. Compositional changes that reduce the surface-to-core lipid ratio of LDL promote LTIP binding and activation.
Figures




References
-
- Morton R. E. 1990. Interaction of lipid transfer protein with plasma lipoproteins and cell membranes. Experientia. 46: 552–560. - PubMed
-
- Tall A. 1995. Plasma lipid transfer proteins. Annu. Rev. Biochem. 64: 235–257. - PubMed
-
- Morton R. E., Steinbrunner J. V. 1990. Concentration of neutral lipids in the phospholipid surface of substrate particles determines lipid transfer protein activity. J. Lipid Res. 31: 1559–1567. - PubMed
-
- Morton R. E., Serdyuk A. P. 1997. Cholesteryl ester transfer protein (CETP) has no preference for cholesteryl esters in high density- versus low density- lipoproteins. Circulation. 96: I-108.
-
- Wang X., Driscoll D. M., Morton R. E. 1999. Molecular cloning and expression of lipid transfer inhibitor protein reveals its identity with apolipoprotein F. J. Biol. Chem. 274: 1814–1820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

