AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function
- PMID: 21937710
- PMCID: PMC3185962
- DOI: 10.1101/gad.17420111
AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function
Abstract
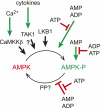
AMP-activated protein kinase (AMPK) is a sensor of energy status that maintains cellular energy homeostasis. It arose very early during eukaryotic evolution, and its ancestral role may have been in the response to starvation. Recent work shows that the kinase is activated by increases not only in AMP, but also in ADP. Although best known for its effects on metabolism, AMPK has many other functions, including regulation of mitochondrial biogenesis and disposal, autophagy, cell polarity, and cell growth and proliferation. Both tumor cells and viruses establish mechanisms to down-regulate AMPK, allowing them to escape its restraining influences on growth.
Figures


References
-
- Amodeo GA, Rudolph MJ, Tong L 2007. Crystal structure of the heterotrimer core of Saccharomyces cerevisiae AMPK homologue SNF1. Nature 449: 492–495 - PubMed
-
- Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC 2004. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116: 457–466 - PubMed
-
- Baena-Gonzalez E, Rolland F, Thevelein JM, Sheen J 2007. A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 - PubMed
-
- Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA 2002. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). J Cell Sci 115: 2433–2442 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
