Peripheral education of the immune system by colonic commensal microbiota
- PMID: 21937990
- PMCID: PMC3192908
- DOI: 10.1038/nature10434
Peripheral education of the immune system by colonic commensal microbiota
Abstract
The instruction of the immune system to be tolerant of self, thereby preventing autoimmunity, is facilitated by the education of T cells in a specialized organ, the thymus, in which self-reactive cells are either eliminated or differentiated into tolerogenic Foxp3(+) regulatory T (T(reg)) cells. However, it is unknown whether T cells are also educated to be tolerant of foreign antigens, such as those from commensal bacteria, to prevent immunopathology such as inflammatory bowel disease. Here we show that encounter with commensal microbiota results in the peripheral generation of T(reg) cells rather than pathogenic effectors. We observed that colonic T(reg) cells used T-cell antigen receptors (TCRs) different from those used by T(reg) cells in other locations, implying an important role for local antigens in shaping the colonic T(reg)-cell population. Many of the local antigens seemed to be derived from commensal bacteria, on the basis of the in vitro reactivity of common colon T(reg) TCRs. These TCRs did not facilitate thymic T(reg)-cell development, implying that many colonic T(reg) cells arise instead by means of antigen-driven peripheral T(reg)-cell development. Further analysis of two of these TCRs by the creation of retroviral bone marrow chimaeras and a TCR transgenic line revealed that microbiota indigenous to our mouse colony was required for the generation of colonic T(reg) cells from otherwise naive T cells. If T cells expressing these TCRs fail to undergo T(reg)-cell development and instead become effector cells, they have the potential to induce colitis, as evidenced by adoptive transfer studies. These results suggest that the efficient peripheral generation of antigen-specific populations of T(reg) cells in response to an individual's microbiota provides important post-thymic education of the immune system to foreign antigens, thereby providing tolerance to commensal microbiota.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
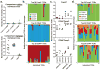
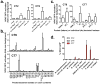


Comment in
-
Mucosal immunology: Colonic creatures are TReg teachers.Nat Rev Immunol. 2011 Oct 25;11(11):721. doi: 10.1038/nri3099. Nat Rev Immunol. 2011. PMID: 22025053 No abstract available.
-
Commensal microbiota determine intestinal iTreg.Am J Transplant. 2012 Aug;12(8):1967. doi: 10.1111/j.1600-6143.2012.04217.x. Am J Transplant. 2012. PMID: 22845904 No abstract available.
-
From infection to colonization: the role of microbiota in transplantation.Am J Transplant. 2013 Apr;13(4):829. doi: 10.1111/ajt.12232. Am J Transplant. 2013. PMID: 23551627 Free PMC article. No abstract available.
References
-
- Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551–89. - PubMed
-
- Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–11. - PubMed
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. - PubMed
-
- Min B, et al. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur J Immunol. 2007;37:1916–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

