Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil siglec-8 expression
- PMID: 21938510
- PMCID: PMC3329870
- DOI: 10.1007/s10875-011-9589-4
Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil siglec-8 expression
Abstract
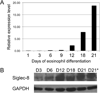
Objective: The aim of this study is to determine when during hematopoiesis Siglec-8 gets expressed, whether it is expressed on hematologic malignancies, and if there are other non-human species that express Siglec-8.
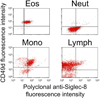
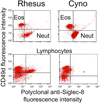
Methods: Siglec-8 mRNA and cell surface expression was monitored during in vitro maturation of human eosinophils and mast cells. Flow cytometry was performed on human blood and bone marrow samples, and on blood samples from dogs, baboons, and rhesus and cynomolgus monkeys.
Results: Siglec-8 is a late maturation marker. It is detectable on eosinophils and basophils from subjects with chronic eosinophilic leukemia, chronic myelogenous leukemia, and on malignant and non-malignant bone marrow mast cells, as well as the HMC-1.2 cell line. None of the Siglec-8 monoclonal antibodies tested recognized leukocytes from dogs, baboons, and rhesus and cynomolgus monkeys.
Conclusions: Siglec-8-based therapies should not target immature human leukocytes but should recognize mature and malignant eosinophils, mast cells, and basophils. So far, there is no suitable species for preclinical testing of Siglec-8 monoclonal antibodies.
Conflict of interest statement
The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Figures
Similar articles
-
An anti-siglec-8 antibody depletes sputum eosinophils from asthmatic subjects and inhibits lung mast cells.Clin Exp Allergy. 2020 Aug;50(8):904-914. doi: 10.1111/cea.13681. Epub 2020 Jul 8. Clin Exp Allergy. 2020. PMID: 32542913 Free PMC article.
-
Sialic acid-binding immunoglobulin-like lectin (Siglec) 8 in patients with eosinophilic disorders: Receptor expression and targeting using chimeric antibodies.J Allergy Clin Immunol. 2019 Jun;143(6):2227-2237.e10. doi: 10.1016/j.jaci.2018.10.066. Epub 2018 Dec 10. J Allergy Clin Immunol. 2019. PMID: 30543818 Free PMC article.
-
Leveraging Siglec-8 endocytic mechanisms to kill human eosinophils and malignant mast cells.J Allergy Clin Immunol. 2018 May;141(5):1774-1785.e7. doi: 10.1016/j.jaci.2017.06.028. Epub 2017 Jul 20. J Allergy Clin Immunol. 2018. PMID: 28734845 Free PMC article.
-
"Siglec"ting the allergic response for therapeutic targeting.Glycobiology. 2016 Jun;26(6):546-52. doi: 10.1093/glycob/cww024. Epub 2016 Feb 23. Glycobiology. 2016. PMID: 26911285 Free PMC article. Review.
-
Discovery, Function, and Therapeutic Targeting of Siglec-8.Cells. 2020 Dec 24;10(1):19. doi: 10.3390/cells10010019. Cells. 2020. PMID: 33374255 Free PMC article. Review.
Cited by
-
Prominent role of IFN-γ in patients with aspirin-exacerbated respiratory disease.J Allergy Clin Immunol. 2013 Oct;132(4):856-65.e1-3. doi: 10.1016/j.jaci.2013.05.008. Epub 2013 Jun 24. J Allergy Clin Immunol. 2013. PMID: 23806637 Free PMC article.
-
Flow cytometric immunophenotypic differentiation patterns of bone marrow eosinophilopoiesis.Cytometry B Clin Cytom. 2024 Sep;106(5):370-382. doi: 10.1002/cyto.b.22174. Epub 2024 Apr 26. Cytometry B Clin Cytom. 2024. PMID: 38666394
-
Human Lung Mast Cells: Therapeutic Implications in Asthma.Int J Mol Sci. 2022 Nov 21;23(22):14466. doi: 10.3390/ijms232214466. Int J Mol Sci. 2022. PMID: 36430941 Free PMC article. Review.
-
First Evidence for a Role of Siglec-8 in Breast Cancer.Int J Mol Sci. 2021 Feb 18;22(4):2000. doi: 10.3390/ijms22042000. Int J Mol Sci. 2021. PMID: 33670444 Free PMC article.
-
AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice.Int Arch Allergy Immunol. 2019;180(2):91-102. doi: 10.1159/000501637. Epub 2019 Aug 9. Int Arch Allergy Immunol. 2019. PMID: 31401630 Free PMC article.
References
-
- Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8: a novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–866. - PubMed
-
- Kikly KK, Bochner BS, Freeman S, Tan KB, Gallagher KT, D'Alessio K, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells and basophils. J Allergy Clin Immunol. 2000;105:1093–1100. - PubMed
-
- Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

