Motility and chemotaxis in Campylobacter and Helicobacter
- PMID: 21939377
- PMCID: PMC6238628
- DOI: 10.1146/annurev-micro-090110-102908
Motility and chemotaxis in Campylobacter and Helicobacter
Abstract
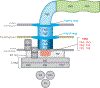
Flagellar motility of Campylobacter jejuni and Helicobacter pylori influences host colonization by promoting migration through viscous milieus such as gastrointestinal mucus. This review explores mechanisms C. jejuni and H. pylori employ to control flagellar biosynthesis and chemotactic responses. These microbes tightly control the activities of σ(54) and σ(28) to mediate ordered flagellar gene expression. In addition to phase-variable and posttranslational mechanisms, flagellar biosynthesis is regulated spatially and numerically so that only a certain number of organelles are placed at polar sites. To mediate chemotaxis, C. jejuni and H. pylori combine basic chemotaxis signal transduction components with several accessory proteins. H. pylori is unusual in that it lacks a methylation-based adaptation system and produces multiple CheV coupling proteins. Chemoreceptors in these bacteria contain nonconserved ligand binding domains, with several chemoreceptors matched to environmental signals. Together, these mechanisms allow for swimming motility that is essential for colonization.
Figures
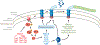

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

