Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia
- PMID: 21939558
- PMCID: PMC3308035
- DOI: 10.1186/scrt79
Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia
Abstract
Introduction: Stem cell transplantation is a promising therapeutic strategy for the treatment of stroke. Mesenchymal stem cells (MSCs) are a potential cell source for clinical application because they can be easily obtained and cultivated with a high proliferative capacity. The safety and efficacy of cell therapy depends on the mode of cell administration. To determine the therapeutic potential of intrathecal administration of MSCs by lumbar puncture (LP), we administrated human umbilical cord blood-derived MSCs (hUCB-MSCs) intrathecally into the lumbar spinal cord or intravenously into the tail vein in a rat model of stroke, and then investigated whether hUCB-MSCs could enter the brain, survive, and improve post-stroke neurological functional recovery.
Methods: hUCB-MSCs (1.0 × 10(6)) were administrated three days after stroke induced by occlusion of the middle cerebral artery. The presence of hUCB-MSCs and their survival and differentiation in the brain tissue of the rats was examined by immunohistochemistry. Recovery of coordination of movement after administration of hUCB-MSCs was examined using a Rotarod test and adhesive-removal test on the 7th, 14th, 21st, and 28th days after ischemia. The volume of ischemic lesions seven days after the experimental procedure was evaluated using 2-3-5-triphenyltetrazolium (TTC) staining.
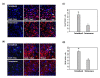
Results: Rats receiving hUCB-MSCs intrathecally by LP had a significantly higher number of migrated cells within the ischemic area when compared with animals receiving cells intravenously. In addition, many of the cells administered intrathecally survived and a subset of them expressed mature neural-lineage markers, including the mature neuron marker NeuN and glial fibrillary acidic protein, typical of astrocytes. Animals that received hUCB-MSCs had significantly improved motor function and reduced ischemic damage when compared with untreated control animals. Regardless of the administration route, the group treated with 1 × 10(6) hUCB-MSCs showed better neurological recovery, without significant differences between the two treatment groups. Importantly, intrathecal administration of 5 × 10(5) hUCB-MSCs significantly reduced ischemic damage, but not in the intravenously treated group. Furthermore, the cells administered intrathecally survived and migrated into the ischemic area more extensively, and differentiated significantly into neurons and astrocytes.
Conclusions: Together, these results indicate that intrathecal administration of MSCs by LP may be useful and feasible for MSCs treatment of brain injuries, such as stroke, or neurodegenerative disorders.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

