Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression
- PMID: 21939614
- PMCID: PMC3534764
- DOI: 10.4088/JCP.10m06634
Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression
Abstract
Objective: Randomized trials of omega-3 polyunsaturated fatty acid (PUFA) treatment for depression have differed in outcome. Recent meta-analyses ascribe discrepancies to differential effects of eicosapentaenoic acid (EPA) versus docosahexaenoic acid (DHA) and to diagnostic heterogeneity. This meta-analysis tests the hypothesis that EPA is the effective component in PUFA treatment of major depressive episodes.
Data sources: PubMed/MeSH was searched for studies published in English from 1960 through June 2010 using the terms fish oils (MeSH) AND (depressive disorder [MeSH] OR bipolar depression) AND randomized controlled trial (publication type). The search was supplemented by manual bibliography review and examination of relevant review articles.
Study selection: The search yielded 15 trials involving 916 participants. Studies were included if they had a prospective, randomized, double-blinded, placebo-controlled study design; if depressive episode was the primary complaint (with or without comorbid medical conditions); if omega-3 PUFA supplements were administered; and if appropriate outcome measures were used to assess depressed mood.
Data extraction: Extracted data included study design, sample sizes, doses and percentages of EPA and DHA, mean ages, baseline and endpoint depression ratings and standard deviations for PUFA and placebo groups, and P values. The clinical outcome of interest was the standardized mean difference in the change from baseline to endpoint scores on a depression rating scale in subjects taking PUFA supplements versus subjects taking placebo.
Data synthesis: In a mixed-effect model, percentage of EPA in the supplements was the fixed-effect predictor, dichotomized into 2 groups: EPA < 60% or EPA ≥ 60% of the total EPA + DHA. Secondary analyses explored the relevance of treatment duration, age, and EPA dose.
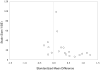
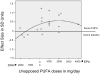
Results: Supplements with EPA ≥ 60% showed benefit on standardized mean depression scores (effect size = 0.532; 95% CI, 0.277-0.733; t = 4.195; P < .001) versus supplements with EPA < 60% (effect size = -0.026; 95% CI, -0.200 to 0.148; t = -0.316; P = .756), with negligible contribution of random effects or heteroscedasticity and with no effects of treatment duration or age. Supplements with EPA < 60% were ineffective. Exploratory analyses supported a nonlinear model, with improvement determined by the dose of EPA in excess of DHA, within the range of 200 to 2,200 mg/d of EPA.
Conclusions: Supplements containing EPA ≥ 60% of total EPA + DHA, in a dose range of 200 to 2,200 mg/d of EPA in excess of DHA, were effective against primary depression. Translational studies are needed to determine the mechanisms of EPA's therapeutic benefit.
© Copyright 2011 Physicians Postgraduate Press, Inc.
Figures

Comment in
-
The impact of omega-3 fatty acids on depressive disorders and suicidality: can we reconcile 2 studies with seemingly contradictory results?J Clin Psychiatry. 2011 Dec;72(12):1574-6. doi: 10.4088/JCP.11com07463. J Clin Psychiatry. 2011. PMID: 22244020 Free PMC article. No abstract available.
References
-
- Lin PY, Huang SY, Su KP. A Meta-Analytic Review of Polyunsaturated Fatty Acid Compositions in Patients with Depression. Biol Psychiatry. 2010;68(2):140–147. - PubMed
-
- McNamara R, Hahn C, Jandacek R, et al. Selective Deficits in the Omega-3 Fatty Acid Docosahexaenoic Acid in the Postmortem Orbitofrontal Cortex of Patients with Major Depressive Disorder. Biol Psychiatry. 2007;62(1):17–24. - PubMed
-
- Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential Fatty Acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163(6):1100–1102. - PubMed
-
- Calder PC. n-3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond) 2004;107(1):1–11. - PubMed
-
- Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354(9177):447–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

