Neutralization of non-vaccine human papillomavirus pseudoviruses from the A7 and A9 species groups by bivalent HPV vaccine sera
- PMID: 21939712
- PMCID: PMC3359499
- DOI: 10.1016/j.vaccine.2011.09.021
Neutralization of non-vaccine human papillomavirus pseudoviruses from the A7 and A9 species groups by bivalent HPV vaccine sera
Abstract
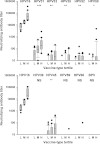
The majority of cervical cancers are associated with infection by one or more Human Papillomavirus (HPV) types from just two distinct Alpha-Papillomavirus species groups, A7 and A9. The extent to which the current HPV16/18 vaccines will protect against other genetically related HPV types is of interest to inform vaccine implementation, cervical disease surveillance and the development of second generation HPV vaccines. The aim of this study was to determine the frequency and titer of neutralizing antibodies against a range of A7 (18, 39, 45, 59, 68) and A9 (16, 31, 33, 35, 52, 58) HPV types using sera from individuals immunized with the bivalent HPV vaccine within the school-based, UK national HPV immunization programme. Serum samples were collected from 69 girls aged 13-14 years, a median 5.9 months (inter-quartile range, IQR, 5.7-6.0) after their third vaccine dose. Cross-neutralizing antibodies against HPV31, HPV33, HPV35 and HPV45 were common and strongly associated with the titer for the related vaccine-type, but were considerably lower (<1%) than their related vaccine type-specific response. The low prevalence of these HPV types in the population and the ages within the study cohort suggest these responses are due to vaccination. It is unclear whether such low levels of neutralizing antibodies would be sufficient to protect at the site of infection in the absence of other immune effectors but the coincidence with HPV types reported from efficacy studies is intriguing. The utility of neutralizing antibodies as surrogate markers of protection remains to be determined.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
References
-
- Munoz N., Bosch F.X., de Sanjose S., Herrero R., Castellsague X., Shah K.V. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. - PubMed
-
- Li N., Franceschi S., Howell-Jones R., Snijders P.J., Clifford G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2010;128(4):927–935. - PubMed
-
- Paavonen J., Naud P., Salmeron J., Wheeler C.M., Chow S.N., Apter D. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. - PubMed
-
- Kjaer S.K., Sigurdsson K., Iversen O.E., Hernandez-Avila M., Wheeler C.M., Perez G. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila) 2009;2(10):868–878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

