Fluid dynamic aspects of ejection in hypertrophic cardiomyopathy
- PMID: 21940289
- PMCID: PMC5788450
Fluid dynamic aspects of ejection in hypertrophic cardiomyopathy
Figures
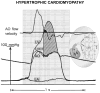
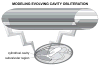
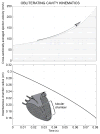
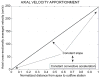

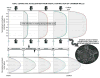
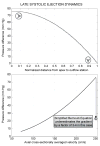

References
-
- Pasipoularides A. Heart’s vortex: intracardiac blood flow phenomena. Shelton, CT: People’s Medical Publishing House; 2010. p. 960. p.-See, in particular, Chapter 8. A gallery of multisensor catheter cardiodynamics, p. 408–41, and Chapter 16. A recapitulation with clinical and basic science perspectives, p. 851–888.
-
- Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults: echocardiographic analysis of 4111 subjects in the CARDIA study. Circulation. 1995;92:785–789. - PubMed
-
- Brock R. Functional obstruction of the left ventricle: acquired aortic subvalvular stenosis. Guy’s Hospital Reports. 1957;106:221–238. - PubMed
-
- Morrow AG, Braunwald E. Functional aortic stenosis; a malformation characterized by resistance to left ventricular outflow without anatomic obstruction. Circulation. 1959;20:181–189. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
