Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis
- PMID: 21940419
- PMCID: PMC3193830
- DOI: 10.1093/cid/cir613
Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis
Abstract
Background: Many deaths from cryptococcal meningitis (CM) may be preventable through early diagnosis and treatment. An inexpensive point-of-care (POC) assay for use with urine or a drop of blood would facilitate early diagnosis of cryptococcal infection in resource-limited settings. We compared cryptococcal antigen (CRAG) concentrations in plasma, serum, and urine from patients with CM, using an antigen-capture assay for glucuronoxylomannan (GXM) and a novel POC dipstick test.
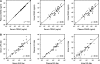
Methods: GXM concentrations were determined in paired serum, plasma, and urine from 62 patients with active or recent CM, using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA). A dipstick lateral-flow assay developed using the same monoclonal antibodies for the sandwich ELISA was tested in parallel. Correlation coefficients were calculated using Spearman rank test.
Results: All patients had detectable GXM in serum, plasma, and urine using the quantitative ELISA. Comparison of paired serum and plasma showed identical results. There were strong correlations between GXM levels in serum/urine (r(s) = 0.86; P < .001) and plasma/urine (r(s) = 0.85; P < .001). Levels of GXM were 22-fold lower in urine than in serum/plasma. The dipstick test was positive in serum, plasma, and urine in 61 of 62 patients. Dipstick titers correlated strongly with ELISA. Correlations between the methods were 0.93 (P < .001) for serum, 0.94 (P < .001) for plasma, and 0.94 (P < .001) for urine.
Conclusions: This novel dipstick test has the potential to markedly improve early diagnosis of CM in many settings, enabling testing of urine in patients presenting to health care facilities in which lumbar puncture, or even blood sampling, is not feasible.
Figures

Comment in
-
Point-of-care urine antigen screening tests for tuberculosis and cryptococcosis: potential for mortality reduction in antiretroviral treatment programs in Africa.Clin Infect Dis. 2012 Mar 1;54(5):739-40. doi: 10.1093/cid/cir908. Epub 2011 Dec 21. Clin Infect Dis. 2012. PMID: 22190561 Free PMC article. No abstract available.
-
Use of the correlation coefficient to compare a point-of-care antigen test against a quantitative sandwich enzyme-linked immunosorbent assay for the detection of cryptococcal meningitis.Clin Infect Dis. 2012 Dec;55(12):1744-5; author reply 1745-6. doi: 10.1093/cid/cis780. Epub 2012 Sep 5. Clin Infect Dis. 2012. PMID: 22955428 Free PMC article. No abstract available.
References
-
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. - PubMed
-
- Bekondi C, Bernede C, Passone N, et al. Primary and opportunistic pathogens associated with meningitis in adults in Bangui, Central African Republic, in relation to human immunodeficiency virus serostatus. Int J Infect Dis. 2006;10:387–95. - PubMed
-
- Bicanic T, Wood R, Meintjes G, et al. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: a randomized trial. Clin Infect Dis. 2008;47:123–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

