Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model
- PMID: 21940438
- PMCID: PMC6623281
- DOI: 10.1523/JNEUROSCI.2156-11.2011
Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model
Abstract
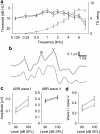
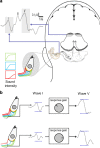
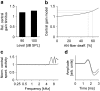
Ever since Pliny the Elder coined the term tinnitus, the perception of sound in the absence of an external sound source has remained enigmatic. Traditional theories assume that tinnitus is triggered by cochlear damage, but many tinnitus patients present with a normal audiogram, i.e., with no direct signs of cochlear damage. Here, we report that in human subjects with tinnitus and a normal audiogram, auditory brainstem responses show a significantly reduced amplitude of the wave I potential (generated by primary auditory nerve fibers) but normal amplitudes of the more centrally generated wave V. This provides direct physiological evidence of "hidden hearing loss" that manifests as reduced neural output from the cochlea, and consequent renormalization of neuronal response magnitude within the brainstem. Employing an established computational model, we demonstrate how tinnitus could arise from a homeostatic response of neurons in the central auditory system to reduced auditory nerve input in the absence of elevated hearing thresholds.
Figures

References
-
- Barnea G, Attias J, Gold S, Shahar A. Tinnitus with normal hearing sensitivity: extended high-frequency audiometry and auditory-nerve brain-stem-evoked responses. Audiology. 1990;29:36–45. - PubMed
-
- Bauer CA, Brozoski TJ, Myers K. Primary afferent dendrite degeneration as a cause of tinnitus. J Neurosci Res. 2007;85:1489–1498. - PubMed
-
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. - PubMed
-
- Dominguez M, Becker S, Bruce I, Read H. A spiking neuron model of cortical correlates of sensorineural hearing loss: Spontaneous firing, synchrony, and tinnitus. Neural Comput. 2006;18:2942–2958. - PubMed
-
- Don M, Eggermont JJ. Analysis of the click-evoked brainstem potentials in man using high-pass noise masking. J Acoust Soc Am. 1978;63:1084–1092. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
