Forebrain CRF₁ modulates early-life stress-programmed cognitive deficits
- PMID: 21940453
- PMCID: PMC3380621
- DOI: 10.1523/JNEUROSCI.2259-11.2011
Forebrain CRF₁ modulates early-life stress-programmed cognitive deficits
Abstract
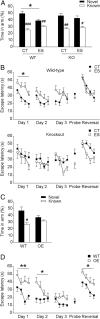
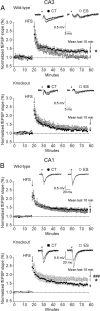
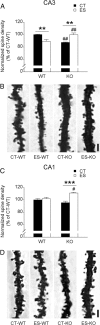
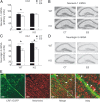
Childhood traumatic events hamper the development of the hippocampus and impair declarative memory in susceptible individuals. Persistent elevations of hippocampal corticotropin-releasing factor (CRF), acting through CRF receptor 1 (CRF₁), in experimental models of early-life stress have suggested a role for this endogenous stress hormone in the resulting structural modifications and cognitive dysfunction. However, direct testing of this possibility has been difficult. In the current study, we subjected conditional forebrain CRF₁ knock-out (CRF₁-CKO) mice to an impoverished postnatal environment and examined the role of forebrain CRF₁ in the long-lasting effects of early-life stress on learning and memory. Early-life stress impaired spatial learning and memory in wild-type mice, and postnatal forebrain CRF overexpression reproduced these deleterious effects. Cognitive deficits in stressed wild-type mice were associated with disrupted long-term potentiation (LTP) and a reduced number of dendritic spines in area CA3 but not in CA1. Forebrain CRF₁ deficiency restored cognitive function, LTP and spine density in area CA3, and augmented CA1 LTP and spine density in stressed mice. In addition, early-life stress differentially regulated the amount of hippocampal excitatory and inhibitory synapses in wild-type and CRF₁-CKO mice, accompanied by alterations in the neurexin-neuroligin complex. These data suggest that the functional, structural and molecular changes evoked by early-life stress are at least partly dependent on persistent forebrain CRF₁ signaling, providing a molecular target for the prevention of cognitive deficits in adults with a history of early-life adversity.
Figures

References
-
- Aisa B, Tordera R, Lasheras B, Del Río J, Ramírez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. - PubMed
-
- Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. - PubMed
-
- Arndt SS, Laarakker MC, van Lith HA, van der Staay FJ, Gieling E, Salomons AR, van't Klooster J, Ohl F. Individual housing of mice—impact on behaviour and stress responses. Physiol Behav. 2009;97:385–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
