Short- and long-term evolutionary dynamics of bacterial insertion sequences: insights from Wolbachia endosymbionts
- PMID: 21940637
- PMCID: PMC3205602
- DOI: 10.1093/gbe/evr096
Short- and long-term evolutionary dynamics of bacterial insertion sequences: insights from Wolbachia endosymbionts
Abstract
Transposable elements (TE) are one of the major driving forces of genome evolution, raising the question of the long-term dynamics underlying their evolutionary success. Long-term TE evolution can readily be reconstructed in eukaryotes, thanks to many degraded copies constituting genomic fossil records of past TE proliferations. By contrast, bacterial genomes usually experience high sequence turnover and short TE retention times, thereby obscuring ancient TE evolutionary patterns. We found that Wolbachia bacterial genomes contain 52-171 insertion sequence (IS) TEs. IS account for 11% of Wolbachia wRi, which is one of the highest IS genomic coverage reported in prokaryotes to date. We show that many IS groups are currently expanding in various Wolbachia genomes and that IS horizontal transfers are frequent among strains, which can explain the apparent synchronicity of these IS proliferations. Remarkably, >70% of Wolbachia IS are nonfunctional. They constitute an unusual bacterial IS genomic fossil record providing direct empirical evidence for a long-term IS evolutionary dynamics following successive periods of intense transpositional activity. Our results show that comprehensive IS annotations have the potential to provide new insights into prokaryote TE evolution and, more generally, prokaryote genome evolution. Indeed, the identification of an important IS genomic fossil record in Wolbachia demonstrates that IS elements are not always of recent origin, contrary to the conventional view of TE evolution in prokaryote genomes. Our results also raise the question whether the abundance of IS fossils is specific to Wolbachia or it may be a general, albeit overlooked, feature of prokaryote genomes.
Figures
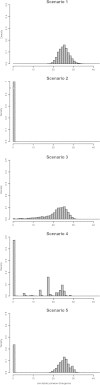
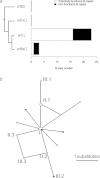

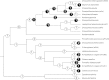
References
-
- Bandelt HJ, Forster P, Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol. 1999;16(1):37–48. - PubMed
-
- Bichsel M, Barbour AD, Wagner A. The early phase of a bacterial insertion sequence infection. Theor Popul Biol. 2010;78(4):278–288. - PubMed
-
- Bordenstein SR, Reznikoff WS. Mobile DNA in obligate intracellular bacteria. Nat Rev Microbiol. 2005;3(9):688–699. - PubMed
-
- Bordenstein SR, Wernegreen JJ. Bacteriophage flux in end (Wolbachia): infection frequency, lateral transfer, and recombination rates. Mol Biol Evol. 2004;21(10):1981–1991. - PubMed
-
- Bouchon D, Cordaux R, Grève P. Feminizing Wolbachia and the evolution of sex determination in isopods. In: Bourtzis K, Miller T, editors. Insect Symbiosis. 2008. Insect Symbiosis, Vol. 3. Boca Raton (FL): Taylor and Francis Group LLC. p. 273–294.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

