Androgen influence on prefrontal dopamine systems in adult male rats: localization of cognate intracellular receptors in medial prefrontal projections to the ventral tegmental area and effects of gonadectomy and hormone replacement on glutamate-stimulated extracellular dopamine level
- PMID: 21940701
- PMCID: PMC3500858
- DOI: 10.1093/cercor/bhr258
Androgen influence on prefrontal dopamine systems in adult male rats: localization of cognate intracellular receptors in medial prefrontal projections to the ventral tegmental area and effects of gonadectomy and hormone replacement on glutamate-stimulated extracellular dopamine level
Abstract
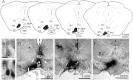
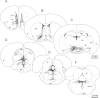
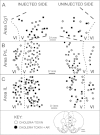
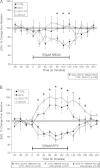
Although androgens are known to modulate dopamine (DA) systems and DA-dependent behaviors of the male prefrontal cortex (PFC), how this occurs remains unclear. Because relatively few ventral tegmental area (VTA) mesoprefrontal DA neurons contain intracellular androgen receptors (ARs), studies presented here combined retrograde tracing and immunolabeling for AR in male rats to determine whether projections afferent to the VTA might be more AR enriched. Results revealed PFC-to-VTA projections to be substantially AR enriched. Because these projections modulate VTA DA cell firing and PFC DA levels, influence over this pathway could be means whereby androgens modulate PFC DA. To assess the hormone sensitivity of glutamate stimulation of PFC DA tone, additional studies utilized microdialysis/reverse dialysis application of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and N-methyl-D-aspartate receptor subtype-selective antagonists which act locally within the PFC and tegmentally via inhibition or disinhibition of PFC-to-VTA afferents to modulate intracortical DA levels. Here, we compared the effects of these drug challenges in control, gonadectomized, and gonadectomized rats given testosterone or estradiol. This revealed complex effects of gonadectomy on antagonist-stimulated PFC DA levels that together with the anatomical data above suggest that androgen stimulation of PFC DA systems does engage glutamatergic circuitry and perhaps that of the AR-enriched glutamatergic projections from PFC-to-VTA specifically.
Figures


References
-
- Adler A, Vescovo P, Robinson JK, Kritzer MF. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89:939–954. - PubMed
-
- Akhondzadeh S, Rezaei F, Larijani B, Nejatisafa AA, Kashani L, Abbasi SH. Correlation between testosterone, gonadotropins and prolactin and severity of negative symptoms in male patients with chronic schizophrenia. Schizophr Res. 2006;84:405–410. - PubMed
-
- Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

