Inhibition of Id1 augments insulin secretion and protects against high-fat diet-induced glucose intolerance
- PMID: 21940780
- PMCID: PMC3178288
- DOI: 10.2337/db11-0083
Inhibition of Id1 augments insulin secretion and protects against high-fat diet-induced glucose intolerance
Abstract
Objective: The molecular mechanisms responsible for pancreatic β-cell dysfunction in type 2 diabetes remain unresolved. Increased expression of the helix-loop-helix protein Id1 has been found in islets of diabetic mice and in vitro models of β-cell dysfunction. Here, we investigated the role of Id1 in insulin secretion and glucose homeostasis.
Research design and methods: Id1 knockout (Id1(-/-)) and wild-type mice were fed a chow or high-fat diet. Glucose tolerance, insulin tolerance, β-cell mass, insulin secretion, and islet gene expression were assessed. Small interfering RNA (siRNA) was used to silence Id1 in MIN6 cells, and responses to chronic palmitate treatment were assessed.
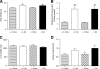
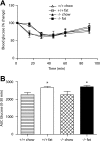
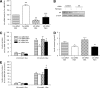
Results: Id1(-/-) mice exhibited an improved response to glucose challenge and were almost completely protected against glucose intolerance induced by high-fat diet. This was associated with increased insulin levels and enhanced insulin release from isolated islets, whereas energy intake, body weight, fat pad weight, β-cell mass, and insulin action were unchanged. Islets from Id1(-/-) mice displayed reduced stress gene expression and were protected against high-fat diet-induced downregulation of β-cell gene expression (pancreatic duodenal homeobox-1, Beta2, Glut2, pyruvate carboxylase, and Gpr40). In MIN6 cells, siRNA-mediated inhibition of Id1 enhanced insulin secretion after chronic palmitate treatment and protected against palmitate-mediated loss of β-cell gene expression.
Conclusions: These findings implicate Id1 as a negative regulator of insulin secretion. Id1 expression plays an essential role in the etiology of glucose intolerance, insulin secretory dysfunction, and β-cell dedifferentiation under conditions of increased lipid supply.
Figures

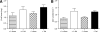

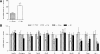
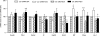
Comment in
-
ID'ing a novel inhibitor of β-cell function, Id1.Diabetes. 2011 Oct;60(10):2455-6. doi: 10.2337/db11-1084. Diabetes. 2011. PMID: 21949000 Free PMC article. No abstract available.
References
-
- Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S. Towards better understanding of the contributions of overwork and glucotoxicity to the beta-cell inadequacy of type 2 diabetes. Diabetes Obes Metab 2009;11(Suppl. 4):82–90 - PubMed
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 - PubMed
-
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008;10(Suppl. 4):32–42 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

