Impact of introducing the pneumococcal and rotavirus vaccines into the routine immunization program in Niger
- PMID: 21940923
- PMCID: PMC3386610
- DOI: 10.2105/AJPH.2011.300218
Impact of introducing the pneumococcal and rotavirus vaccines into the routine immunization program in Niger
Abstract
Objectives: We investigated whether introducing the rotavirus and pneumococcal vaccines, which are greatly needed in West Africa, would overwhelm existing supply chains (i.e., the series of steps required to get a vaccine from the manufacturers to the target population) in Niger.
Methods: As part of the Bill and Melinda Gates Foundation-funded Vaccine Modeling Initiative, we developed a computational model to determine the impact of introducing these new vaccines to Niger's Expanded Program on Immunization vaccine supply chain.
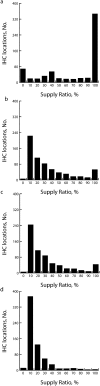
Results: Introducing either the rotavirus vaccine or the 7-valent pneumococcal conjugate vaccine could overwhelm available storage and transport refrigerator space, creating bottlenecks that would prevent the flow of vaccines down to the clinics. As a result, the availability of all World Health Organization Expanded Program on Immunization vaccines to patients might decrease from an average of 69% to 28.2% (range = 10%-51%). Addition of refrigerator and transport capacity could alleviate this bottleneck.
Conclusions: Our results suggest that the effects on the vaccine supply chain should be considered when introducing a new vaccine and that computational models can help assess evolving needs and prevent problems with vaccine delivery.
Figures



References
-
- Naghipour M, Nakagomi T, Nakagomi O. Issues with reducing the rotavirus-associated mortality by vaccination in developing countries. Vaccine. 2008;26(26):3236–3241 - PubMed
-
- Scott JAG. The preventable burden of pneumococcal disease in the developing world. Vaccine. 2007;25(13):2398–2405 - PubMed
-
- Clemens J, Jodar L. Introducing new vaccines into developing countries: obstacles, opportunities and complexities. Nat Med. 2005;11(4 suppl):S12–S15 - PubMed
-
- de Oliveira LH, Danovaro-Holliday MC, Matus CR, Andrus JK. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert Rev Vaccines. 2008;7(3):345–353 - PubMed
-
- World Health Organiation Immunization profile—Niger. Available at: http://apps.who.int/immunization_monitoring/en/globalsummary/countryprof.... Accessed October 27, 2010
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

