Organ-specific features of natural killer cells
- PMID: 21941294
- PMCID: PMC3620656
- DOI: 10.1038/nri3065
Organ-specific features of natural killer cells
Erratum in
- Nat Rev Immunol. 2011 Dec;11(12):880
Abstract
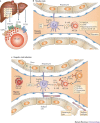
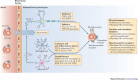
Natural killer (NK) cells can be swiftly mobilized by danger signals and are among the earliest arrivals at target organs of disease. However, the role of NK cells in mounting inflammatory responses is often complex and sometimes paradoxical. Here, we examine the divergent phenotypic and functional features of NK cells, as deduced largely from experimental mouse models of pathophysiological responses in the liver, mucosal tissues, uterus, pancreas, joints and brain. Moreover, we discuss how organ-specific factors, the local microenvironment and unique cellular interactions may influence the organ-specific properties of NK cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Shi F-D, Ransohoff RM. Natural Killer Cells. 2010. pp. 373–383.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

