The AMPK stress response pathway mediates anoikis resistance through inhibition of mTOR and suppression of protein synthesis
- PMID: 21941369
- PMCID: PMC3278733
- DOI: 10.1038/cdd.2011.119
The AMPK stress response pathway mediates anoikis resistance through inhibition of mTOR and suppression of protein synthesis
Abstract
Suppression of anoikis after detachment of cancer cells from the extracellular matrix is a key step during metastasis. Here we show that, after detachment, mouse embryonic fibroblasts (MEFs) transformed by K-Ras(V12) or ETV6-NTRK3 (EN) activate a transcriptional response overrepresented by genes related to bioenergetic stress and the AMP-activated protein kinase (AMPK) energy-sensing pathway. Accordingly, AMPK is activated in both transformed and non-transformed cells after detachment, and AMPK deficiency restores anoikis to transformed MEFs. However, AMPK activation represses the mTOR complex-1 (mTORC1) pathway only in transformed cells, suggesting a key role for AMPK-mediated mTORC1 inhibition in the suppression of anoikis. Consistent with this, AMPK-/- MEFs transformed by EN or K-Ras show sustained mTORC1 activation after detachment and fail to suppress anoikis. Transformed TSC1-/- MEFs, which are incapable of suppressing mTORC1, also undergo anoikis after detachment, which is reversed by mTORC1 inhibitors. Furthermore, transformed AMPK-/- and TSC1-/- MEFs both have higher total protein synthesis rates than wild-type controls, and translation inhibition using cycloheximide partially restores their anoikis resistance, indicating a mechanism whereby mTORC1 inhibition suppresses anoikis. Finally, breast carcinoma cell lines show similar detachment-induced AMPK/mTORC1 activation and restoration of anoikis by AMPK inhibition. Our data implicate AMPK-mediated mTORC1 inhibition and suppression of protein synthesis as a means for bioenergetic conservation during detachment, thus promoting anoikis resistance.
Figures

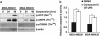
References
-
- Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–185. - PubMed
-
- Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. - PubMed
-
- Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

