Easy and rapid purification of highly active nisin
- PMID: 21941571
- PMCID: PMC3175705
- DOI: 10.1155/2011/175145
Easy and rapid purification of highly active nisin
Abstract
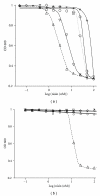
Nisin is an antimicrobial peptide produced and secreted by several L. lactis strains and is specifically active against Gram-positive bacteria. In previous studies, nisin was purified via cation exchange chromatography at low pH employing a single-step elution using 1 M NaCl. Here, we describe an optimized purification protocol using a five-step NaCl elution to remove contaminants. The obtained nisin is devoid of impurities and shows high bactericidal activity against the nisin-sensitive L. lactis strain NZ9000. Purified nisin exhibits an IC(50) of ~3 nM, which is a tenfold improvement as compared to nisin obtained via the one-step elution procedure.
Figures




References
-
- Klaenhammer TR. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiology Reviews. 1993;12(1-3):39–85. - PubMed
-
- Chatterjee C, Paul M, Xie L, van der Donk WA. Biosynthesis and mode of action of lantibiotics. Chemical Reviews. 2005;105(2):633–683. - PubMed
-
- Enserink M. Promising antibiotic candidate identified. Science. 1999;286(5448):2245–2247. - PubMed
-
- Kuipers OP, Beerthuyzen MM, Siezen RJ, De Vos WM. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for the development of immunity. European Journal of Biochemistry. 1993;216(1):281–291. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

