Kinetic Characterisation of Phosphofructokinase Purified from Setaria cervi: A Bovine Filarial Parasite
- PMID: 21941634
- PMCID: PMC3173978
- DOI: 10.4061/2011/939472
Kinetic Characterisation of Phosphofructokinase Purified from Setaria cervi: A Bovine Filarial Parasite
Abstract
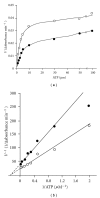
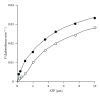
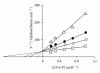
Phosphofructokinase (PFK), a regulatory enzyme in glycolytic pathway, has been purified to electrophoretic homogeneity from adult female Setaria cervi and partially characterized. For this enzyme, the Lineweaver-Burk's double reciprocal plots of initial rates and D-fructose-6-phosphate (F-6-P) or Mg-ATP concentrations for varying values of cosubstrate concentration gave intersecting lines indicating that K(m) values for F-6-P (1.05 mM) and ATP (3 μM) were independent of each other. S. cervi PFK, when assayed at inhibitory concentration of ATP (>0.1 mM), exhibited sigmoidal behavior towards binding with F-6-P with a Hill coefficient (n) value equal to 1.8 and 1.7 at 1.0 and 0.33 mM ATP, respectively. D-fructose-1,6-diphosphate (FDP) competitively inhibited the filarial enzyme: K(i) and Hill coefficient values being 0.18 μM and 2.0, respectively. Phosphoenolpyruvate (PEP) also inhibited the enzyme competitively with the K(i) value equal to 0.8 mM. The Hill coefficient values (>1.5) for F-6-P (at inhibitory concentration of ATP) and FDP suggested its positive cooperative kinetics towards F-6-P and FDP, showing presence of more than one binding sites for these molecules in enzyme protein and allosteric nature of the filarial enzyme. The product inhibition studies gave us the only compatible mechanism of random addition process with a probable orientation of substrates and products on the enzyme surface.
Figures





References
-
- MacDonald JA, Storey KB. Reassessment of the cold-labile nature of phosphofructokinase from a hibernating ground squirrel. Molecular and Cellular Biochemistry. 2001;225(1-2):51–57. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

