Human norovirus infection of caco-2 cells grown as a three-dimensional tissue structure
- PMID: 21942189
- PMCID: PMC3187569
- DOI: 10.2166/wh.2010.106
Human norovirus infection of caco-2 cells grown as a three-dimensional tissue structure
Abstract
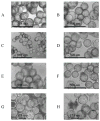
Human norovirus (hNoV) infectivity was studied using a three-dimensional model of large intestinal epithelium. Large intestine Caco-2 cells were grown in rotating wall vessel bioreactors for 18-21 days at 37 degrees C and then transferred to 24-well tissue culture plates where they were infected with GI.1 and GII.4 human noroviruses collected from human challenge trials and various outbreak settings, respectively. Compared with uninfected cells, transmission micrographs of norovirus-infected cells displayed evidence of shortening or total loss of apical microvilli, and vacuolization. Quantitative reverse transcription real-time PCR (qRT-PCR) indicated an approximate 2-3 log10 increase in viral RNA copies for the infected cells. A passage experiment examined both the ability for continued viral RNA and viral antigen detection. In the passaged samples 1.01x10(6) copies ml(-1) were detected by qRT-PCR. Immune electron microscopy using primary antibody to hNoV GI.1 capsids in conjunction with 6 nm gold-labelled secondary antibodies was performed on crude cellular lysates. Localization of antibody was observed in infected but not for uninfected cells. Our present findings, coupled with earlier work with the three-dimensional small intestinal INT407 model, demonstrate the utility of 3-D cell culture methods to develop infectivity assays for enteric viruses that do not readily infect mammalian cell cultures.
Figures



References
-
- Carvalho HM, Teel LD, Goping G, O’Brien AD. A three dimensional tissue culture model for the study of attach and efface lesion formation by enteropathogenic and enterohaemorrhagic Esherichia coli. Cell Microbiol. 2005;7:1771–1781. - PubMed
-
- Centers for Disease Control and Prevention (U.S.) Norovirus activity - United States, 2006-2007. MMWR Morb Mortal Wkly Rep. 2007;56(33):842–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

