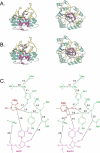
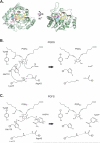
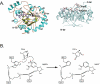
Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis
- PMID: 21942677
- PMCID: PMC3285496
- DOI: 10.1021/cr2002992
Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis
Figures
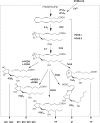



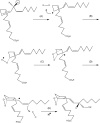
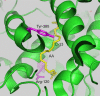
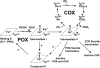

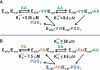
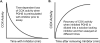



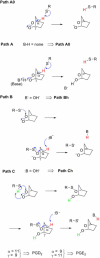
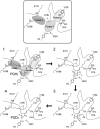


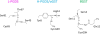
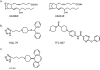


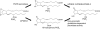
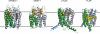

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

