Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data
- PMID: 21942989
- PMCID: PMC3206450
- DOI: 10.1186/1471-2326-11-4
Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data
Abstract
Background: Recommendations given for intravenous iron treatment are typically not supported by a high level of evidence. This meta-analysis addressed this by summarising the available date from clinical trials of ferric carboxymaltose using clinical trial reports and published reports.
Methods: Clinical trial reports were supplemented by electronic literature searches comparing ferric carboxymaltose with active comparators or placebo. Various outcomes were sought for efficacy (attainment of normal haemoglobin (Hb), increase of Hb by a defined amount, for example), together with measures of harm, including serious adverse events and deaths.
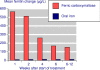
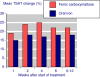
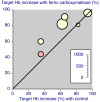
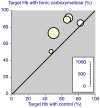
Results: Fourteen studies were identified with 2,348 randomised patients exposed to ferric carboxymaltose, 832 to oral iron, 762 to placebo, and 384 to intravenous iron sucrose. Additional data were available from cohort studies. Intravenous ferric carboxymaltose was given up to the calculated iron deficit (up to 1,000 mg in one week) for iron deficiency anaemia secondary to chronic kidney disease, blood loss in obstetric and gynaecological conditions, gastrointestinal disease, and other conditions like heart failure. The most common comparator was oral iron, and trials lasted 1 to 24 weeks. Intravenous ferric carboxymaltose improved mean Hb, serum ferritin, and transferrin saturation levels; the mean end-of-trial increase over oral iron was, for Hb 4.8 (95% confidence interval 3.3 to 6.3) g/L, for ferritin 163 (153 to 173) μg/L, and for transferrin saturation 5.3% (3.7 to 6.8%). Ferric carboxymaltose was significantly better than comparator in achievement of target Hb increase (number needed to treat (NNT) 6.8; 5.3 to 9.7) and target Hb NNT (5.9; 4.7 to 8.1). Serious adverse events and deaths were similar in incidence in ferric carboxymaltose and comparators; rates of constipation, diarrhoea, and nausea or vomiting were lower than with oral iron.
Conclusions: This review examined the available trials of intravenous ferric carboxymaltose using details from published papers and unpublished clinical trial reports. It increases the evidence available to support recommendations given for intravenous iron treatment, but there are limited trial data comparing different intravenous iron preparations.
Figures




References
-
- de Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993-2005. WHO Global Database on Anaemia, World Health Organisation; 2008.
-
- National Institute for Health and Clinical Excellence. Clinical guideline 39: Anaemia management in people with chronic kidney disease (CKD) 2006. http://guidance.nice.org.uk/CG39 (last accessed February 3 2011)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

