A holistic phylogeny of the coronin gene family reveals an ancient origin of the tandem-coronin, defines a new subfamily, and predicts protein function
- PMID: 21943019
- PMCID: PMC3203266
- DOI: 10.1186/1471-2148-11-268
A holistic phylogeny of the coronin gene family reveals an ancient origin of the tandem-coronin, defines a new subfamily, and predicts protein function
Abstract
Background: Coronins belong to the superfamily of the eukaryotic-specific WD40-repeat proteins and play a role in several actin-dependent processes like cytokinesis, cell motility, phagocytosis, and vesicular trafficking. Two major types of coronins are known: First, the short coronins consisting of an N-terminal coronin domain, a unique region and a short coiled-coil region, and secondly the tandem coronins comprising two coronin domains.
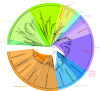
Results: 723 coronin proteins from 358 species have been identified by analyzing the whole-genome assemblies of all available sequenced eukaryotes (March 2011). The organisms analyzed represent most eukaryotic kingdoms but also cover every taxon several times to provide a better statistical sampling. The phylogenetic tree of the coronin domains based on the Bayesian method is in accordance with the most recent grouping of the major kingdoms of the eukaryotes and also with the grouping of more recently separated branches. Based on this "holistic" approach the coronins group into four classes: class-1 (Type I) and class-2 (Type II) are metazoan/choanoflagellate specific classes, class-3 contains the tandem-coronins (Type III), and the new class-4 represents the coronins fused to villin (Type IV). Short coronins from non-metazoans are equally related to class-1 and class-2 coronins and thus remain unclassified.
Conclusions: The coronin class distribution suggests that the last common eukaryotic ancestor possessed a single and a tandem-coronin, and most probably a class-4 coronin of which homologs have been identified in Excavata and Opisthokonts although most of these species subsequently lost the class-4 homolog. The most ancient short coronin already contained the trimerization motif in the coiled-coil domain.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

