A novel angiopoietin-derived peptide displays anti-angiogenic activity and inhibits tumour-induced and retinal neovascularization
- PMID: 21943108
- PMCID: PMC3372838
- DOI: 10.1111/j.1476-5381.2011.01677.x
A novel angiopoietin-derived peptide displays anti-angiogenic activity and inhibits tumour-induced and retinal neovascularization
Abstract
Background and purpose: Pathological angiogenesis is associated with various human diseases, such as cancer, autoimmune diseases and retinopathy. The angiopoietin (Ang)-Tie2 system plays critical roles in several steps of angiogenic remodelling. Here, we have investigated the anti-angiogenic effect of a novel angiopoietin-derived peptide.
Experimental approach: Using computational methods, we identified peptides from helical segments within angiopoietins, which were predicted to inhibit their activity. These peptides were tested using biochemical methods and models of angiogenesis. The peptide with best efficacy, A11, was selected for further characterization as an anti-angiogenic compound.
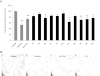
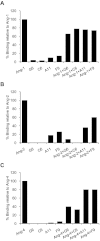
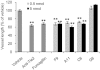
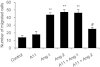
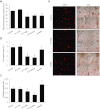
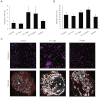
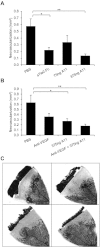
Key results: The potent anti-angiogenic activity of A11 was demonstrated in a multicellular assay of angiogenesis and in the chorioallantoic membrane model. A11 bound to angiopoietins and reduced the binding of Ang-2 to Tie2. A11 was also significantly reduced vascular density in a model of tumour-induced angiogenesis. Its ability to inhibit Ang-2 but not Ang-1-induced endothelial cell migration, and to down-regulate Tie2 levels in tumour microvessels, suggests that A11 targets the Ang-Tie2 pathway. In a rat model of oxygen-induced retinopathy, A11 strongly inhibited retinal angiogenesis. Moreover, combination of A11 with an anti-VEGF antibody showed a trend for further inhibition of angiogenesis, suggesting an additive effect.
Conclusions and implications: Our results indicate that A11 is a potent anti-angiogenic compound, through modulation of the Ang-Tie2 system, underlining its potential as a therapeutic agent for the treatment of ocular and tumour neovascularization, as well as other pathological conditions that are dependent on angiogenesis.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
References
-
- Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. - PubMed
-
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. - PubMed
-
- Bishop ET, Bell GT, Bloor S, Broom IJ, Hendry NF, Wheatley DN. An in vitro model of angiogenesis: basic features. Angiogenesis. 1999;3:335–344. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

