Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis
- PMID: 21943360
- PMCID: PMC3203098
- DOI: 10.1186/1471-2407-11-404
Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis
Abstract
Background: Febrile neutropenia (FN) occurs following myelosuppressive chemotherapy and is associated with morbidity, mortality, costs, and chemotherapy reductions and delays. Granulocyte colony-stimulating factors (G-CSFs) stimulate neutrophil production and may reduce FN incidence when given prophylactically following chemotherapy.
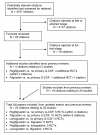
Methods: A systematic review and meta-analysis assessed the effectiveness of G-CSFs (pegfilgrastim, filgrastim or lenograstim) in reducing FN incidence in adults undergoing chemotherapy for solid tumours or lymphoma. G-CSFs were compared with no primary G-CSF prophylaxis and with one another. Nine databases were searched in December 2009. Meta-analysis used a random effects model due to heterogeneity.
Results: Twenty studies compared primary G-CSF prophylaxis with no primary G-CSF prophylaxis: five studies of pegfilgrastim; ten of filgrastim; and five of lenograstim. All three G-CSFs significantly reduced FN incidence, with relative risks of 0.30 (95% CI: 0.14 to 0.65) for pegfilgrastim, 0.57 (95% CI: 0.48 to 0.69) for filgrastim, and 0.62 (95% CI: 0.44 to 0.88) for lenograstim. Overall, the relative risk of FN for any primary G-CSF prophylaxis versus no primary G-CSF prophylaxis was 0.51 (95% CI: 0.41 to 0.62). In terms of comparisons between different G-CSFs, five studies compared pegfilgrastim with filgrastim. FN incidence was significantly lower for pegfilgrastim than filgrastim, with a relative risk of 0.66 (95% CI: 0.44 to 0.98).
Conclusions: Primary prophylaxis with G-CSFs significantly reduces FN incidence in adults undergoing chemotherapy for solid tumours or lymphoma. Pegfilgrastim reduces FN incidence to a significantly greater extent than filgrastim.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

