ColVI myopathies: where do we stand, where do we go?
- PMID: 21943391
- PMCID: PMC3189202
- DOI: 10.1186/2044-5040-1-30
ColVI myopathies: where do we stand, where do we go?
Abstract
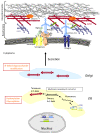
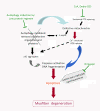
Collagen VI myopathies, caused by mutations in the genes encoding collagen type VI (ColVI), represent a clinical continuum with Ullrich congenital muscular dystrophy (UCMD) and Bethlem myopathy (BM) at each end of the spectrum, and less well-defined intermediate phenotypes in between. ColVI myopathies also share common features with other disorders associated with prominent muscle contractures, making differential diagnosis difficult. This group of disorders, under-recognized for a long time, has aroused much interest over the past decade, with important advances made in understanding its molecular pathogenesis. Indeed, numerous mutations have now been reported in the COL6A1, COL6A2 and COL6A3 genes, a large proportion of which are de novo and exert dominant-negative effects. Genotype-phenotype correlations have also started to emerge, which reflect the various pathogenic mechanisms at play in these disorders: dominant de novo exon splicing that enables the synthesis and secretion of mutant tetramers and homozygous nonsense mutations that lead to premature termination of translation and complete loss of function are associated with early-onset, severe phenotypes. In this review, we present the current state of diagnosis and research in the field of ColVI myopathies. The past decade has provided significant advances, with the identification of altered cellular functions in animal models of ColVI myopathies and in patient samples. In particular, mitochondrial dysfunction and a defect in the autophagic clearance system of skeletal muscle have recently been reported, thereby opening potential therapeutic avenues.
Figures

References
-
- Bidanset D, Guidry C, Rosenberg L, Choi H, Timpl R, Hook M. Binding of the proteoglycan decorin to collagen type VI. J Biol Chem. 1992;267:5250–5256. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

