Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo
- PMID: 21943605
- PMCID: PMC3183990
- DOI: 10.1016/j.neuron.2011.08.007
Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo
Abstract
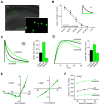
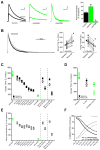
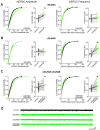
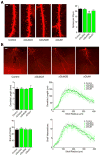
During development there is an activity-dependent switch in synaptic N-Methyl-D-aspartate (NMDA) receptor subunit composition from predominantly GluN2B to GluN2A, though the precise role of this switch remains unknown. By deleting GluN2 subunits in single neurons during synaptogenesis, we find that both GluN2B and GluN2A suppress AMPA receptor expression, albeit by distinct means. Similar to GluN1, GluN2B deletion increases the number of functional synapses, while GluN2A deletion increases the strength of unitary connections without affecting the number of functional synapses. We propose a model of excitatory synapse maturation in which baseline activation of GluN2B-containing receptors prevents premature synapse maturation until correlated activity allows induction of functional synapses. This activity also triggers the switch to GluN2A, which dampens further potentiation. Furthermore, we analyze the subunit composition of synaptic NMDA receptors in CA1 pyramidal cells, provide electrophysiological evidence for a large population of synaptic triheteromeric receptors, and estimate the subunit-dependent open probability.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Akashi K, Kakizaki T, Kamiya H, Fukaya M, Yamasaki M, Abe M, Natsume R, Watanabe M, Sakimura K. NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J Neurosci. 2009;29:10869–10882. - PMC - PubMed
-
- Al-Hallaq RA, Jarabek BR, Fu Z, Vicini S, Wolfe BB, Yasuda RP. Association of NR3A with the N-methyl-D-aspartate receptor NR1 and NR2 subunits. Mol Pharmacol. 2002;62:1119–1127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

