Mutations in iron-sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of multiple respiratory chain and 2-oxoacid dehydrogenase enzymes
- PMID: 21944046
- PMCID: PMC3188835
- DOI: 10.1016/j.ajhg.2011.08.011
Mutations in iron-sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of multiple respiratory chain and 2-oxoacid dehydrogenase enzymes
Abstract
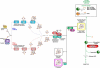
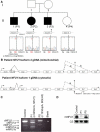
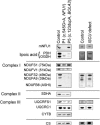
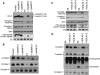
Severe combined deficiency of the 2-oxoacid dehydrogenases, associated with a defect in lipoate synthesis and accompanied by defects in complexes I, II, and III of the mitochondrial respiratory chain, is a rare autosomal recessive syndrome with no obvious causative gene defect. A candidate locus for this syndrome was mapped to chromosomal region 2p14 by microcell-mediated chromosome transfer in two unrelated families. Unexpectedly, analysis of genes in this area identified mutations in two different genes, both of which are involved in [Fe-S] cluster biogenesis. A homozygous missense mutation, c.545G>A, near the splice donor of exon 6 in NFU1 predicting a p.Arg182Gln substitution was found in one of the families. The mutation results in abnormal mRNA splicing of exon 6, and no mature protein could be detected in fibroblast mitochondria. A single base-pair duplication c.123dupA was identified in BOLA3 in the second family, causing a frame shift that produces a premature stop codon (p.Glu42Argfs(∗)13). Transduction of fibroblast lines with retroviral vectors expressing the mitochondrial, but not the cytosolic isoform of NFU1 and with isoform 1, but not isoform 2 of BOLA3 restored both respiratory chain function and oxoacid dehydrogenase complexes. NFU1 was previously proposed to be an alternative scaffold to ISCU for the biogenesis of [Fe-S] centers in mitochondria, and the function of BOLA3 was previously unknown. Our results demonstrate that both play essential roles in the production of [Fe-S] centers for the normal maturation of lipoate-containing 2-oxoacid dehydrogenases, and for the assembly of the respiratory chain complexes.
Copyright © 2011 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

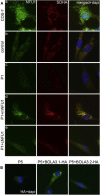
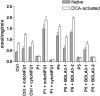
References
-
- Cameron J.M., Levandovskiy V., Mackay N., Tein I., Robinson B.H. Deficiency of pyruvate dehydrogenase caused by novel and known mutations in the E1alpha subunit. Am. J. Med. Genet. A. 2004;131:59–66. - PubMed
-
- Lissens W., De Meirleir L., Seneca S., Liebaers I., Brown G.K., Brown R.M., Ito M., Naito E., Kuroda Y., Kerr D.S. Mutations in the X-linked pyruvate dehydrogenase (E1) alpha subunit gene (PDHA1) in patients with a pyruvate dehydrogenase complex deficiency. Hum. Mutat. 2000;15:209–219. - PubMed
-
- Kerr D.S., Wexler I.D., Tripatara A., Patel M.S. Human defects of the pyruvate dehydrogenase complex. In: Patel M.S., Harris R.A., editors. Alpha-keto acid dehydrogenase complexes, R.T. Birkhäuser; Basel: 1996. pp. 249–269.
-
- Maj M.C., MacKay N., Levandovskiy V., Addis J., Baumgartner E.R., Baumgartner M.R., Robinson B.H., Cameron J.M. Pyruvate dehydrogenase phosphatase deficiency: Identification of the first mutation in two brothers and restoration of activity by protein complementation. J. Clin. Endocrinol. Metab. 2005;90:4101–4107. - PubMed
-
- Robinson B.H. Lactic acidemia (Disorders of pyruvate carboxylase, pyruvate dehydrogenase) In: Scriver C.R., Sly W.S., Valle D., editors. The metabolic and molecular bases of inherited disease, B.A. McGraw-Hill; New York: 2001. pp. 2275–2295.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

