Acetylcholine from the mesopontine tegmental nuclei differentially affects methamphetamine induced locomotor activity and neurotransmitter levels in the mesolimbic pathway
- PMID: 21945297
- PMCID: PMC3270566
- DOI: 10.1016/j.bbr.2011.09.022
Acetylcholine from the mesopontine tegmental nuclei differentially affects methamphetamine induced locomotor activity and neurotransmitter levels in the mesolimbic pathway
Abstract
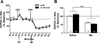
Methamphetamine (MA) increases dopamine (DA) levels within the mesolimbic pathway and acetylcholine (ACh), a neurotransmitter known to increase DA cell firing and release and mediate reinforcement, within the ventral tegmental area (VTA). The laterodorsal tegmental (LDT) and pedunculopontine tegmental (PPT) nuclei provide cholinergic input to the VTA; however, the contribution of LDT- and PPT-derived ACh to MA-induced DA and ACh levels and locomotor activation remains unknown. The first experiment examined the role of LDT-derived ACh in MA locomotor activation by reversibly inhibiting these neurons with bilateral intra-LDT microinjections of the M2 receptor agonist oxotremorine (OXO). Male C57BL/6J mice were given a bilateral 0.1μl OXO (0, 1, or 10nM/side) microinjection immediately prior to IP saline or MA (2mg/kg). The highest OXO concentration significantly inhibited both saline- and MA-primed locomotor activity. In a second set of experiments we characterized the individual contributions of ACh originating in the LDT or pedunculopontine tegmental nucleus (PPT) to MA-induced levels of ACh and DA by administering intra-LDT or PPT OXO and performing in vivo microdialysis in the VTA and NAc. Intra-LDT OXO dose-dependently attenuated the MA-induced increase in ACh within the VTA but had no effect on DA in NAc. Intra-PPT OXO had no effect on ACh or DA levels within the VTA or NAc, respectively. We conclude that LDT, but not PPT, ACh is important in locomotor behavior and the cholinergic, but not dopaminergic, response to systemic MA.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures






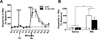
References
-
- Winn P. How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J Neurol Sci. 2006;248:234–250. - PubMed
-
- Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596–3604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

