LMNA mutations induce a non-inflammatory fibrosis and a brown fat-like dystrophy of enlarged cervical adipose tissue
- PMID: 21945321
- PMCID: PMC3204020
- DOI: 10.1016/j.ajpath.2011.07.049
LMNA mutations induce a non-inflammatory fibrosis and a brown fat-like dystrophy of enlarged cervical adipose tissue
Abstract
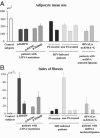
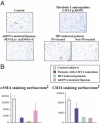
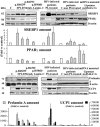
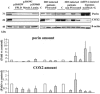
Some LMNA mutations responsible for insulin-resistant lipodystrophic syndromes are associated with peripheral subcutaneous lipoatrophy and faciocervical fat accumulation. Their pathophysiologic characteristics are unknown. We compared histologic, immunohistologic, ultrastructural, and protein expression features of enlarged cervical subcutaneous adipose tissue (scAT) obtained during plastic surgery from four patients with LMNA p.R482W, p.R439C, or p.H506D mutations versus cervical fat from eight control subjects, buffalo humps from five patients with HIV infection treated or not with protease inhibitors, and dorsocervical lipomas from two patients with mitochondrial DNA mutations. LMNA-mutated cervical scAT and HIV-related buffalo humps were dystrophic, with an increased percentage of small adipocytes, increased fibrosis without inflammatory features, and decreased number of blood vessels, as compared with control samples. Samples from patients with LMNA mutations or protease inhibitor-based therapy demonstrated accumulation of prelamin A, altered expression of adipogenic proteins and brown fat-like features, with an increased number of mitochondria and overexpression of uncoupling protein 1 (UCP1). These features were absent in samples from control subjects and from patients with HIV not treated with protease inhibitors. Mitochondrial DNA-mutated cervical lipomas demonstrated inflammatory fibrosis with distinct mitochondrial abnormalities but neither UCP1 expression nor prelamin A accumulation. In conclusion, Enlarged cervical scAT from patients with lipodystrophy demonstrated small adipocytes, fibrosis, and decreased vessel numbers. However, only cervical fat from patients with LMNA mutations or who had received protease inhibitor therapy accumulated prelamin A and exhibited similar remodeling toward a brown-like phenotype with UCP1 overexpression and mitochondrial alterations.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Mattout A., Dechat T., Adam S.A., Goldman R.D., Gruenbaum Y. Nuclear lamins, diseases and aging. Curr Opin Cell Biol. 2006;18:335–341. - PubMed
-
- Cao H., Hegele R.A. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2000;9:109–112. - PubMed
-
- Shackleton S., Lloyd D.J., Jackson S.N., Evans R., Niermeijer M.F., Singh B.M., Schmidt H., Brabant G., Kumar S., Durrington P.N., Gregory S., O'Rahilly S., Trembath R.C. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet. 2000;24:153–156. - PubMed
-
- Vigouroux C., Caron-Debarle M., Le Dour C., Magré J., Capeau J. Molecular mechanisms of human lipodystrophies: from adipocyte lipid droplet to oxidative stress and lipotoxicity. Int J Biochem Cell Biol. 2011;43:862–876. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

