VEGFR-1 mediates endothelial differentiation and formation of blood vessels in a murine model of infantile hemangioma
- PMID: 21945324
- PMCID: PMC3204018
- DOI: 10.1016/j.ajpath.2011.07.040
VEGFR-1 mediates endothelial differentiation and formation of blood vessels in a murine model of infantile hemangioma
Abstract
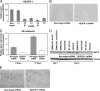
Vascular endothelial growth factor receptor-1 (VEGFR-1) is a member of the VEGFR family, and binds to VEGF-A, VEGF-B, and placental growth factor. VEGFR-1 contributes to tumor growth and metastasis, but its role in the pathological formation of blood vessels is still poorly understood. Herein, we used infantile hemangioma (IH), the most common tumor of infancy, as a means to study VEGFR-1 activation in pathological vasculogenesis. IH arises from stem cells (HemSCs) that can form the three most prominent cell types in the tumor: endothelial cells, pericytes, and adipocytes. HemSCs can recapitulate the IH life cycle when injected in immuncompromised mice, and are targeted by corticosteroids, the traditional treatment for IH. We report here that VEGF-A or VEGF-B induces VEGFR-1-mediated ERK1/2 phosphorylation in HemSCs and promotes differentiation of HemSCs to endothelial cells. Studies of VEGFR-2 phosphorylation status and down-regulation of neuropilin-1 in the HemSCs demonstrate that VEGFR-2 and NRP1 are not needed for VEGF-A- or VEGF-B-induced ERK1/2 activation. U0216-mediated blockade of ERK1/2 phosphorylation or shRNA-mediated suppression of VEGFR-1 prevents HemSC-to-EC differentiation. Furthermore, the down-regulation of VEGFR-1 in the HemSCs results in decreased formation of blood vessels in vivo and reduced ERK1/2 activation. Thus, our study reveals a critical role for VEGFR-1 in the HemSC-to-EC differentiation that underpins pathological vasculogenesis in IH.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




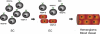
References
-
- Fong G.H., Rossant J., Gertsenstein M., Breitman M.L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. - PubMed
-
- Hiratsuka S., Maru Y., Okada A., Seiki M., Noda T., Shibuya M. Involvement of Flt-1 tyrosine kinase (vascular endothelial growth factor receptor-1) in pathological angiogenesis. Cancer Res. 2001;61:1207–1213. - PubMed
-
- Kaplan R.N., Riba R.D., Zacharoulis S., Bramley A.H., Vincent L., Costa C., MacDonald D.D., Jin D.K., Shido K., Kerns S.A., Zhu Z., Hicklin D., Wu Y., Port J.L., Altorki N., Port E.R., Ruggero D., Shmelkov S.V., Jensen K.K., Rafii S., Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. - PMC - PubMed
-
- Fischer C., Mazzone M., Jonckx B., Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. - PubMed
-
- Carmeliet P., Ferreira V., Breier G., Pollefeyt S., Kieckens L., Gertsenstein M., Fahrig M., Vandenhoeck A., Harpal K., Eberhardt C., Declercq C., Pawling J., Moons L., Collen D., Risau W., Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

