Depressive phenotypes evoked by experimental diabetes are reversed by insulin
- PMID: 21945451
- PMCID: PMC3237925
- DOI: 10.1016/j.physbeh.2011.09.003
Depressive phenotypes evoked by experimental diabetes are reversed by insulin
Abstract
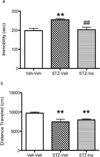
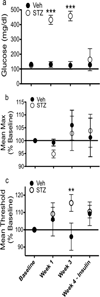
Clinical studies suggest a bidirectional relationship between diabetes and depression, where diabetes may increase risk for depressive symptoms and depression may increase risk for diabetes. Preclinical models examining the effects of diabetes on brain and behavior can provide insights to the pathophysiology underlying this relationship. The current study comprehensively examined, in C57BL/6 mice, the development of depressive phenotypes evoked by diabetes induced by streptozotocin (STZ) and determined if insulin treatment was able to reverse the diabetes-related changes on brain and affective behavior. Since anxiety is often comorbid with mood disturbances, behavioral tests for both anxiety and depression were administered. Possible physiological correlates of behavioral changes, including hippocampal cell proliferation, brain derived neurotrophic factor, and plasma corticosterone, were also measured. STZ-induced diabetes resulted in increased immobility in the tail suspension test, increased intracranial self-stimulation thresholds, decreased hippocampal cell proliferation, and increased corticosterone levels. Insulin treatment, on the other hand, reduced hyperglycemia, reversed the behavioral effects, and returned hippocampal cell proliferation and corticosterone to levels comparable to the control group. Anxiety-related behaviors were unaffected. This study showed that experimental diabetes in the mouse produced depressive phenotypes that were reversed by insulin therapy. Changes in reward-related behaviors and hippocampal cell proliferation may be useful markers to identify therapeutic interventions for comorbid diabetes and depression.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care. 2002;25:464–470. - PubMed
-
- Adili F, Larijani B, Haghighatpanah M. Diabetic patients: Psychological aspects. Ann N Y Acad Sci. 2006;1084:329–349. - PubMed
-
- Egede LE. Disease-focused or integrated treatment: diabetes and depression. Med Clin North Am. 2006;90:627–646. - PubMed
-
- Iosifescu DV. Treating depression in the medically ill. Psychiatr Clin North Am. 2007;30:77–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

