Substance P induces CCN1 expression via histone deacetylase activity in human colonic epithelial cells
- PMID: 21945803
- PMCID: PMC3204086
- DOI: 10.1016/j.ajpath.2011.07.038
Substance P induces CCN1 expression via histone deacetylase activity in human colonic epithelial cells
Abstract
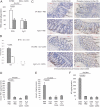
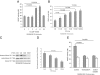
We have shown that substance P (SP) and its neurokinin-1 receptor (NK-1R) regulate intestinal angiogenesis by increasing expression of protein CYR61 (the cysteine-rich angiogenic inducer 61, or CCN1) in colonic epithelial cells. However, the mechanism involved in SP-induced CCN1 expression has not been studied, and the outcome of increased CCN1 expression in the development of colitis is not fully understood. Because histone deacetylase (HDAC) modulates transcription of several genes involved in inflammation, we investigated participation of HDAC in SP-induced CCN1 expression in human colonic epithelial NCM460 cells overexpressing NK-1R (NCM460-NK-1R) and in primary colonocytes. SP increased HDAC activity with deacetylation and dephosphorylation of nucleosome protein histone H3 in NCM460-NK-1R and/or primary colonocytes. Histone deacetylation and dephosphorylation was observed in colonic mucosa from irritable bowel disease patients. Similarly, colonic mucosal tissues from mice exposed to dextran sulfate sodium showed histone H3 deacetylation and dephosphorylation and increased HDAC activity that was reversed by the NK-1R antagonist CJ-12255. SP-induced increased CCN1 expression in NCM460-NK-1R cells was abolished by pharmacological HDAC inhibition. HDAC overexpression activated basal and SP-induced CCN1 promoter activity. Intracolonic CCN1 overexpression significantly ameliorated dextran sulfate sodium-induced colitis, with reduction of proinflammatory cytokine expression in mice. Thus, SP-mediated CCN1 expression in the inflamed human and mouse colon involves increased HDAC activity. Our results strongly suggest that increased CCN1 expression may be involved in mucosal healing during colitis.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Chang M.M., Leeman S.E. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem. 1970;245:4784–4790. - PubMed
-
- Mantyh P.W. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002;63(Suppl 11):6–10. - PubMed
-
- Costa M., Furness J.B., Franco R., Llewellyn-Smith I., Murphy R., Beardsley A.M. Substance P in nerve tissue in the gut. Ciba Found Symp. 1982:129–144. - PubMed
-
- Maggi C.A. Capsaicin-sensitive nerves in the gastrointestinal tract. Arch Int Pharmacodyn Ther. 1990;303:157–166. - PubMed
-
- Bost K.L. Quantification of macrophage-derived substance P receptor mRNA using competitive polymerase chain reaction. Adv Exp Med Biol. 1995;373:219–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

