Chk2-mediated G2/M cell cycle arrest maintains radiation resistance in malignant meningioma cells
- PMID: 21945852
- PMCID: PMC3196767
- DOI: 10.1016/j.canlet.2011.08.022
Chk2-mediated G2/M cell cycle arrest maintains radiation resistance in malignant meningioma cells
Erratum in
- Cancer Lett. 2014 Jul 28;349(2):155
Abstract
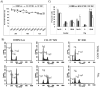
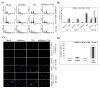
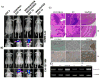
In continuation to our studies on radioresistance in meningioma, here we show that radiation treatment (7Gy) induces G2/M cell cycle arrest in meningioma cells. Phosphorylation of Chk2, Cdc25c and Cdc2 were found to be key events since interference with Chk2 activation and cyclin B1/Cdc2 interaction led to permanent arrest followed by apoptosis. Irradiated cells showed recovery and formed aggressive intracranial tumors with rapid spread and morbidity. Nevertheless, knock down of uPAR with or without radiation induced permanent arrest in G2/M phase and subsequent apoptosis in vitro and in vivo. In conclusion, our data suggest that combination treatment with radiation and uPAR knock down or other inhibitors resulting in non-reversible G2/M arrest may be beneficial in the management of meningiomas.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures





Similar articles
-
siRNA-mediated downregulation of MMP-9 and uPAR in combination with radiation induces G2/M cell-cycle arrest in Medulloblastoma.Mol Cancer Res. 2011 Jan;9(1):51-66. doi: 10.1158/1541-7786.MCR-10-0399. Epub 2010 Dec 10. Mol Cancer Res. 2011. Retraction in: Mol Cancer Res. 2021 Jul;19(7):1247. doi: 10.1158/1541-7786.MCR-21-0395. PMID: 21148633 Free PMC article. Retracted.
-
Elevated cyclin G2 expression intersects with DNA damage checkpoint signaling and is required for a potent G2/M checkpoint arrest response to doxorubicin.J Biol Chem. 2012 Jun 29;287(27):22838-53. doi: 10.1074/jbc.M112.376855. Epub 2012 May 15. J Biol Chem. 2012. PMID: 22589537 Free PMC article.
-
Gallic acid induces G2/M phase cell cycle arrest via regulating 14-3-3β release from Cdc25C and Chk2 activation in human bladder transitional carcinoma cells.Mol Nutr Food Res. 2010 Dec;54(12):1781-90. doi: 10.1002/mnfr.201000096. Mol Nutr Food Res. 2010. PMID: 20564478
-
6,7-Dimethoxy-3-(3-methoxyphenyl)isoquinolin-1-amine induces mitotic arrest and apoptotic cell death through the activation of spindle assembly checkpoint in human cervical cancer cells.Carcinogenesis. 2013 Aug;34(8):1852-60. doi: 10.1093/carcin/bgt133. Epub 2013 Apr 24. Carcinogenesis. 2013. PMID: 23615402
-
Regulation of the G2/M transition by p53.Oncogene. 2001 Apr 5;20(15):1803-15. doi: 10.1038/sj.onc.1204252. Oncogene. 2001. PMID: 11313928 Review.
Cited by
-
MicroRNA-221/222 Inhibits the Radiation-Induced Invasiveness and Promotes the Radiosensitivity of Malignant Meningioma Cells.Front Oncol. 2020 Aug 25;10:1441. doi: 10.3389/fonc.2020.01441. eCollection 2020. Front Oncol. 2020. PMID: 32983973 Free PMC article.
-
Cordycepin enhances radiosensitivity to induce apoptosis through cell cycle arrest, caspase pathway and ER stress in MA-10 mouse Leydig tumor cells.Am J Cancer Res. 2022 Aug 15;12(8):3601-3624. eCollection 2022. Am J Cancer Res. 2022. PMID: 36119830 Free PMC article.
-
Novel insight into metabolic reprogrammming in cancer radioresistance: A promising therapeutic target in radiotherapy.Int J Biol Sci. 2023 Jan 1;19(3):811-828. doi: 10.7150/ijbs.79928. eCollection 2023. Int J Biol Sci. 2023. PMID: 36778122 Free PMC article. Review.
-
Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance.Cell Death Dis. 2013 Oct 24;4(10):e875. doi: 10.1038/cddis.2013.407. Cell Death Dis. 2013. PMID: 24157869 Free PMC article.
-
Targeting MicroRNAs in Prostate Cancer Radiotherapy.Theranostics. 2017 Jul 23;7(13):3243-3259. doi: 10.7150/thno.19934. eCollection 2017. Theranostics. 2017. PMID: 28900507 Free PMC article. Review.
References
-
- Alexiou GA, Gogou P, Markoula S, Kyritsis AP. Management of meningiomas. Clin Neurol Neurosurg. 2010;112:177–182. - PubMed
-
- Norden AD, Drappatz J, Wen PY. Advances in meningioma therapy. Curr Neurol Neurosci Rep. 2009;9:231–240. - PubMed
-
- Kondziolka D, Mathieu D, Lunsford LD, Martin JJ, Madhok R, Niranjan A, Flickinger JC. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62:53–58. - PubMed
-
- Malik I, Rowe JG, Walton L, Radatz MW, Kemeny AA. The use of stereotactic radiosurgery in the management of meningiomas. Br J Neurosurg. 2005;19:13–20. - PubMed
-
- Milker-Zabel S, Zabel-du BA, Huber P, Schlegel W, Debus J. Intensity-modulated radiotherapy for complex-shaped meningioma of the skull base: long-term experience of a single institution. Int J Radiat Oncol Biol Phys. 2007;68:858–863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

