Truth and consequences of sphingosine-1-phosphate lyase
- PMID: 21946005
- PMCID: PMC3560305
- DOI: 10.1016/j.advenzreg.2011.09.015
Truth and consequences of sphingosine-1-phosphate lyase
Abstract
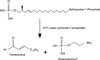
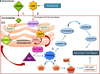
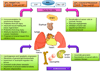
Sphingosine phosphate lyase (SPL) is an intracellular enzyme responsible for the irreversible catabolism of the lipid signaling molecule sphingosine-1-phosphate (S1P). SPL catalyzes the cleavage of S1P resulting in the formation of hexadecenal and ethanolamine phosphate. S1P functions as a ligand for a family of ubiquitously expressed G protein-coupled receptors that mediate autocrine and paracrine signals controlling cell migration, proliferation and programmed cell death pathways. S1P has also been implicated in developmental and pathological angiogenesis, cancer, inflammation, allergy, diabetes, lymphocyte trafficking and morphogenesis of the heart, kidney and brain as well as their response to ischemic injury.
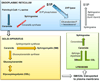
As the final enzyme in the sphingolipid degradative pathway, SPL commands the only exit point for sphingolipid intermediates and their flow into phospholipid metabolism. So, in addition to regulating S1P levels, SPL is the gatekeeper of a critical node of lipid metabolic flow. The recent crystallization of a prokaryotic SPL has provided insight into the function and potential regulation and drug targeting of this enzyme. Considering the many physiological and pathological functions of S1P signaling, it seems likely that targeting SPL to modulate S1P signaling could be useful in a variety of clinical contexts.
In this review we discuss the recent highlights related to SPL-mediated biology, the structure of the SPL protein, the function of its products, new insights regarding the usefulness of SPL targeting in treating human diseases and the consequences of permanent SPL disruption in mice.
Figures

References
-
- Alexander S, Alexander H. Lead genetic studies in Dictyostelium discoideum and translational studies in human cells demonstrate that sphingolipids are key regulators of sensitivity to cisplatin and other anticancer drugs. Semin Cell Dev Biol. 2011;22:97–104. - PubMed
-
- Bagdanoff J, Donoviel M, Nouraldeen A, Carlsen M, Jessop T, Tarver J, et al. Inhibition of sphingosine 1-Phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone Oxime (LX2931) and (1R,2S,3R)-1-(2-(Isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932) J Med Chem. 2010;53:8650–8652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

