Catabolic and anabolic actions of parathyroid hormone on the skeleton
- PMID: 21946081
- PMCID: PMC4315330
- DOI: 10.3275/7925
Catabolic and anabolic actions of parathyroid hormone on the skeleton
Abstract
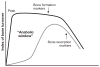
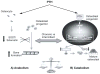
PTH, an 84-amino acid peptide hormone synthesized by the parathyroid glands, is essential for the maintenance of calcium homeostasis.While in its traditional metabolic role, PTH helps to maintain the serum calcium concentration within narrow, normal limits and participates as a determinant of bone remodeling, more specific actions, described as catabolic and anabolic are also well known. Clinically, the catabolic effect of PTH is best represented by primary hyperparathyroidism (PHPT), while the osteoanabolic effect of PTH is best seen when PTH or its biological amino-terminal fragment [PTH(1-34)] is used as a therapy for osteoporosis. These dual functions of PTH are unmasked under very specific pathological (PHPT) or therapeutic conditions. At the cellular level, PTH favors bone resorption, mostly by affecting the receptor activator of nuclear factor κ-B (RANK) ligand (RANKL)-osteoprotegerin- RANK system, leading to an increase in osteoclast formation and activity. Increased bone formation due to PTH therapy is explained best by its ability to enhance osteoblastogenesis and/or osteoblast survival. The PTH-induced bone formation is mediated, in part, by a decrease in SOST/sclerostin expression in osteocytes. This review focuses on the dual anabolic and catabolic actions of PTH on bone, situations where one is enhanced over the other, and the cellular and molecular mechanisms by which these actions are mediated.
Figures
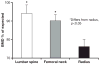


References
-
- Hanley DA, Watson PH, Hodsman AB, Dempster DW. Pharmacological Mechanisms of Therapeutics: Parathyroid Hormone. In: Bilezikian J, Raisz LG, Martin TJ, editors. Principles of Bone Biology. Amsterdam: Elsevier; 2008. pp. 1661–95.
-
- Kousteni S, Bilezikian JP. The cell biology of parathyroid hormone in osteoblasts. Curr Osteoporos Rep. 2008;6:72–6. - PubMed
-
- Potts JT. Parathyroid hormone: past and present. J Endocrinol. 2005;187:311–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

