Cell-free synthetic biology: thinking outside the cell
- PMID: 21946161
- PMCID: PMC3322310
- DOI: 10.1016/j.ymben.2011.09.002
Cell-free synthetic biology: thinking outside the cell
Abstract
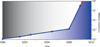
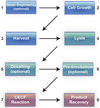
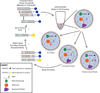
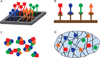
Cell-free synthetic biology is emerging as a powerful approach aimed to understand, harness, and expand the capabilities of natural biological systems without using intact cells. Cell-free systems bypass cell walls and remove genetic regulation to enable direct access to the inner workings of the cell. The unprecedented level of control and freedom of design, relative to in vivo systems, has inspired the rapid development of engineering foundations for cell-free systems in recent years. These efforts have led to programmed circuits, spatially organized pathways, co-activated catalytic ensembles, rational optimization of synthetic multi-enzyme pathways, and linear scalability from the micro-liter to the 100-liter scale. It is now clear that cell-free systems offer a versatile test-bed for understanding why nature's designs work the way they do and also for enabling biosynthetic routes to novel chemicals, sustainable fuels, and new classes of tunable materials. While challenges remain, the emergence of cell-free systems is poised to open the way to novel products that until now have been impractical, if not impossible, to produce by other means.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Alper H, et al. Engineering yeast transcription machinery for improved ethanol tolerance and production. Science. 2006;314:1565–1568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

