The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth
- PMID: 21946849
- PMCID: PMC3240886
- DOI: 10.1113/jphysiol.2011.218255
The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth
Abstract
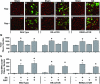
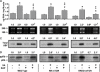
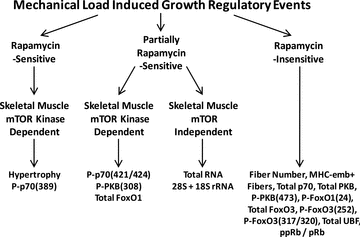
Chronic mechanical loading (CML) of skeletal muscle induces compensatory growth and the drug rapamycin has been reported to block this effect. Since rapamycin is considered to be a highly specific inhibitor of the mammalian target of rapamycin (mTOR), many have concluded that mTOR plays a key role in CML-induced growth regulatory events. However, rapamycin can exert mTOR-independent actions and systemic administration of rapamycin will inhibit mTOR signalling in all cells throughout the body. Thus, it is not clear if the growth inhibitory effects of rapamycin are actually due to the inhibition of mTOR signalling, and more specifically, the inhibition of mTOR signalling in skeletal muscle cells. To address this issue, transgenic mice with muscle specific expression of various rapamycin-resistant mutants of mTOR were employed. These mice enabled us to demonstrate that mTOR, within skeletal muscle cells, is the rapamycin-sensitive element that confers CML-induced hypertrophy, and mTOR kinase activity is necessary for this event. Surprisingly, CML also induced hyperplasia, but this occurred through a rapamycin-insensitive mechanism. Furthermore, CML was found to induce an increase in FoxO1 expression and PKB phosphorylation through a mechanism that was at least partially regulated by an mTOR kinase-dependent mechanism. Finally, CML stimulated ribosomal RNA accumulation and rapamycin partially inhibited this effect; however, the effect of rapamycin was exerted through a mechanism that was independent of mTOR in skeletal muscle cells. Overall, these results demonstrate that CML activates several growth regulatory events, but only a few (e.g. hypertrophy) are fully dependent on mTOR signalling within the skeletal muscle cells.
Figures




References
-
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PRJ, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. - PubMed
-
- Avila G, Lee EH, Perez CF, Allen PD, Dirksen RT. FKBP12 binding to RyR1 modulates excitation-contraction coupling in mouse skeletal myotubes. J Biol Chem. 2003;278:22 600–22 608. - PubMed
-
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. - PubMed
-
- Bentzinger CF, Romanino K, Cloëtta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Rüegg MA. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell metabolism. 2008;8:411–424. - PubMed
-
- Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc. 2006;38:1950–1957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

