Visual motion integration by neurons in the middle temporal area of a New World monkey, the marmoset
- PMID: 21946851
- PMCID: PMC3249047
- DOI: 10.1113/jphysiol.2011.213520
Visual motion integration by neurons in the middle temporal area of a New World monkey, the marmoset
Abstract
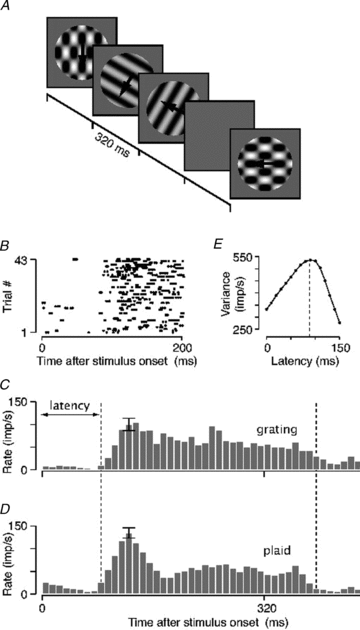
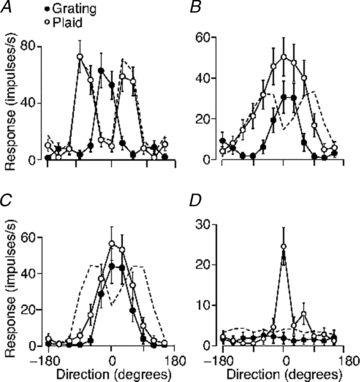
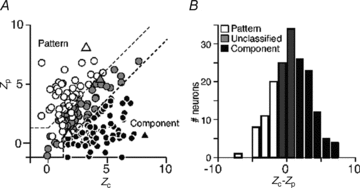
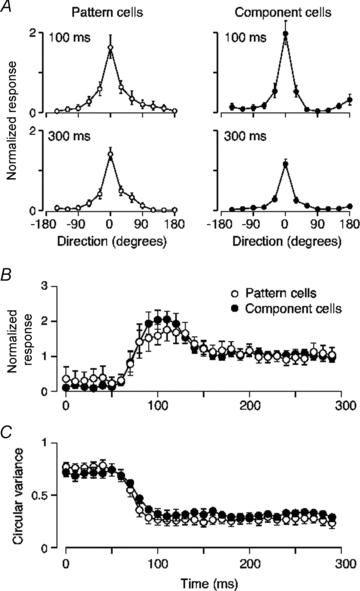
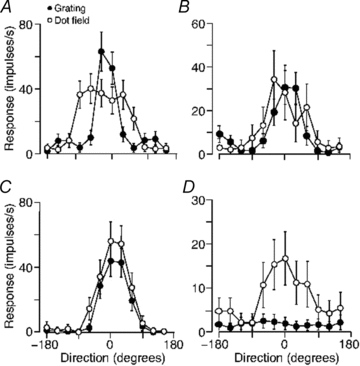
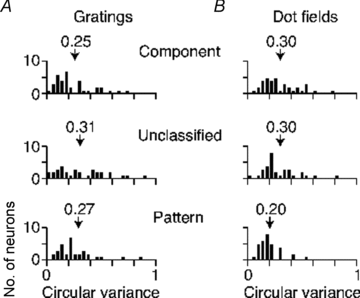
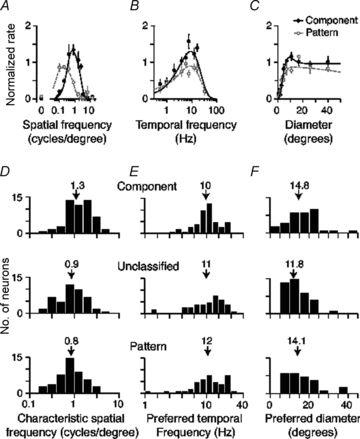
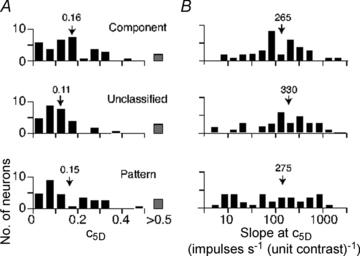
The middle temporal area (MT/V5) is an anatomically distinct region of primate visual cortex that is specialized for the processing of image motion. It is generally thought that some neurons in area MT are capable of signalling the motion of complex patterns, but this has only been established in the macaque monkey. We made extracellular recordings from single units in area MT of anaesthetized marmosets, a New World monkey. We show through quantitative analyses that some neurons (35 of 185; 19%) are capable of signalling pattern motion ('pattern cells'). Across several dimensions, the visual response of pattern cells in marmosets is indistinguishable from that of pattern cells in macaques. Other neurons respond to the motion of oriented contours in a pattern ('component cells') or show intermediate properties. In addition, we encountered a subset of neurons (22 of 185; 12%) insensitive to sinusoidal gratings but very responsive to plaids and other two-dimensional patterns and otherwise indistinguishable from pattern cells. We compared the response of each cell class to drifting gratings and dot fields. In pattern cells, directional selectivity was similar for gratings and dot fields; in component cells, directional selectivity was weaker for dot fields than gratings. Pattern cells were more likely to have stronger suppressive surrounds, prefer lower spatial frequencies and prefer higher speeds than component cells. We conclude that pattern motion sensitivity is a feature of some neurons in area MT of both New and Old World monkeys, suggesting that this functional property is an important stage in motion analysis and is likely to be conserved in humans.
Figures



Similar articles
-
Temporal and spatial limits of pattern motion sensitivity in macaque MT neurons.J Neurophysiol. 2015 Apr 1;113(7):1977-88. doi: 10.1152/jn.00597.2014. Epub 2014 Dec 24. J Neurophysiol. 2015. PMID: 25540222 Free PMC article.
-
Properties of pattern and component direction-selective cells in area MT of the macaque.J Neurophysiol. 2016 Jun 1;115(6):2705-20. doi: 10.1152/jn.00639.2014. Epub 2015 Nov 11. J Neurophysiol. 2016. PMID: 26561603 Free PMC article.
-
Texture-dependent motion signals in primate middle temporal area.J Physiol. 2013 Nov 15;591(22):5671-90. doi: 10.1113/jphysiol.2013.257568. Epub 2013 Sep 2. J Physiol. 2013. PMID: 24000175 Free PMC article.
-
Processing of first- and second-order motion signals by neurons in area MT of the macaque monkey.Vis Neurosci. 1998 Mar-Apr;15(2):305-17. doi: 10.1017/s0952523898152094. Vis Neurosci. 1998. PMID: 9605531
-
Structure and function of the middle temporal visual area (MT) in the marmoset: Comparisons with the macaque monkey.Neurosci Res. 2015 Apr;93:62-71. doi: 10.1016/j.neures.2014.09.012. Epub 2014 Oct 7. Neurosci Res. 2015. PMID: 25304293 Review.
Cited by
-
Ferrets as a Model for Higher-Level Visual Motion Processing.Curr Biol. 2019 Jan 21;29(2):179-191.e5. doi: 10.1016/j.cub.2018.11.017. Epub 2018 Dec 27. Curr Biol. 2019. PMID: 30595516 Free PMC article.
-
The marmoset monkey as a model for visual neuroscience.Neurosci Res. 2015 Apr;93:20-46. doi: 10.1016/j.neures.2015.01.008. Epub 2015 Feb 13. Neurosci Res. 2015. PMID: 25683292 Free PMC article. Review.
-
Temporal and spatial limits of pattern motion sensitivity in macaque MT neurons.J Neurophysiol. 2015 Apr 1;113(7):1977-88. doi: 10.1152/jn.00597.2014. Epub 2014 Dec 24. J Neurophysiol. 2015. PMID: 25540222 Free PMC article.
-
Ocular following Eye Movements in Marmosets Follow Complex Motion Trajectories.eNeuro. 2023 Jun 12;10(6):ENEURO.0072-23.2023. doi: 10.1523/ENEURO.0072-23.2023. Print 2023 Jun. eNeuro. 2023. PMID: 37236785 Free PMC article.
-
Sensitivity of neurons in the middle temporal area of marmoset monkeys to random dot motion.J Neurophysiol. 2017 Sep 1;118(3):1567-1580. doi: 10.1152/jn.00065.2017. Epub 2017 Jun 21. J Neurophysiol. 2017. PMID: 28637812 Free PMC article.
References
-
- Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. J Opt Soc Am A. 1985;2:284–299. - PubMed
-
- Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. J Neurophysiol. 1984;52:1106–1130. - PubMed
-
- Allman J, Miezin F, McGuinness E. Direction- and velocity-specific responses from beyond the classical receptive field in the middle temporal visual area (MT) Perception. 1985;14:105–126. - PubMed
-
- Allman JM, Kaas JH. A representation of the visual field in the caudal third of the middle tempral gyrus of the owl monkey (Aotus trivirgatus) Brain Res. 1971;31:85–105. - PubMed
-
- Baker JF, Petersen SE, Newsome WT, Allman JM. Visual response properties of neurons in four extrastriate visual areas of the owl monkey (Aotus trivirgatus): a quantitative comparison of medial, dorsomedial, dorsolateral, and middle temporal areas. J Neurophysiol. 1981;45:397–416. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

