Liver precursor cells increase hepatic fibrosis induced by chronic carbon tetrachloride intoxication in rats
- PMID: 21946857
- PMCID: PMC3425737
- DOI: 10.1038/labinvest.2011.143
Liver precursor cells increase hepatic fibrosis induced by chronic carbon tetrachloride intoxication in rats
Abstract
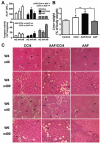
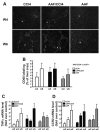
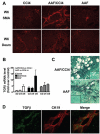
Hepatic fibrosis, the major complication of virtually all types of chronic liver damage, usually begins in portal areas, and its severity has been correlated to liver progenitor cells (LPC) expansion from periportal areas, even if the primary targets of injury are intralobular hepatocytes. The aim of this study was to determine the potential fibrogenic role of LPC, using a new experimental model in which rat liver fibrosis was induced by chronic carbon tetrachloride (CCl(4)) administration for 6 weeks, in combination with chronic acetylaminofluorene treatment (AAF), which promotes activation of LPC compartment. Treatment with CCl(4) alone caused a significant increase in serum transaminase activity as well as liver fibrosis initiating around central veins and leading to formation of incomplete centro-central septa with sparse fibrogenic cells expressing α-smooth muscle actin (αSMA). In AAF/CCl(4)-treated animals, the fibrogenic response was profoundly worsened, with formation of multiple porto-central bridging septa leading to cirrhosis, whereas hepatocellular necrosis and inflammation were similar to those observed in CCl(4)-treated animals. Enhanced fibrosis in AAF/CCl(4) group was accompanied by ductule forming LPC expanding from portal areas, αSMA-positive cells accumulation in the fibrotic areas and increased expression of hepatic collagen type 1, 3 and 4 mRNA. Moreover, CK19-positive LPC expressed the most potent fibrogenic cytokine transforming growth factor-β (TGFβ) without any expression of αSMA, desmin or fibroblast-specific protein-1, demonstrating that LPC did not undergo an epithelial-mesenchymal transition. In this new experimental model, LPC, by expressing TGFβ, contributed to the accumulation of αSMA-positive myofibroblasts in the ductular reaction leading to enhanced fibrosis but also to disease progression and to a fibrotic pattern similar to that observed in humans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Participation of liver progenitor cells in liver regeneration: lack of evidence in the AAF/PH rat model.Lab Invest. 2012 Jan;92(1):72-81. doi: 10.1038/labinvest.2011.136. Epub 2011 Sep 12. Lab Invest. 2012. PMID: 21912377
-
Effects of extract from Ginkgo biloba on carbon tetrachloride-induced liver injury in rats.World J Gastroenterol. 2006 Jun 28;12(24):3924-8. doi: 10.3748/wjg.v12.i24.3924. World J Gastroenterol. 2006. PMID: 16804984 Free PMC article.
-
Estrogen reduces CCL4- induced liver fibrosis in rats.World J Gastroenterol. 2002 Oct;8(5):883-7. doi: 10.3748/wjg.v8.i5.883. World J Gastroenterol. 2002. PMID: 12378635 Free PMC article.
-
(Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione protects rats from carbon tetrachloride-induced liver injury and fibrogenesis.World J Gastroenterol. 2012 Feb 21;18(7):654-61. doi: 10.3748/wjg.v18.i7.654. World J Gastroenterol. 2012. PMID: 22363136 Free PMC article.
-
A review of liver fibrosis and cirrhosis regression.J Pathol Transl Med. 2023 Jul;57(4):189-195. doi: 10.4132/jptm.2023.05.24. Epub 2023 Jun 20. J Pathol Transl Med. 2023. PMID: 37461143 Free PMC article. Review.
Cited by
-
Dynamic expression of desmin, α-SMA and TGF-β1 during hepatic fibrogenesis induced by selective bile duct ligation in young rats.Braz J Med Biol Res. 2014 Oct;47(10):850-7. doi: 10.1590/1414-431x20143679. Epub 2014 Aug 15. Braz J Med Biol Res. 2014. PMID: 25140817 Free PMC article.
-
Systems toxicology of chemically induced liver and kidney injuries: histopathology-associated gene co-expression modules.J Appl Toxicol. 2016 Sep;36(9):1137-49. doi: 10.1002/jat.3278. Epub 2016 Jan 4. J Appl Toxicol. 2016. PMID: 26725466 Free PMC article.
-
Lipopolysaccharide induces the differentiation of hepatic progenitor cells into myofibroblasts via activation of the Hedgehog signaling pathway.Cell Cycle. 2017 Jul 18;16(14):1357-1365. doi: 10.1080/15384101.2017.1325976. Epub 2017 May 31. Cell Cycle. 2017. PMID: 28562206 Free PMC article.
-
TGF-β1 Induces the Dual Regulation of Hepatic Progenitor Cells with Both Anti- and Proliver Fibrosis.Stem Cells Int. 2016;2016:1492694. doi: 10.1155/2016/1492694. Epub 2015 Dec 29. Stem Cells Int. 2016. PMID: 26839553 Free PMC article.
-
M1 Muscarinic Receptor Deficiency Attenuates Azoxymethane-Induced Chronic Liver Injury in Mice.Sci Rep. 2015 Sep 16;5:14110. doi: 10.1038/srep14110. Sci Rep. 2015. PMID: 26374068 Free PMC article.
References
-
- Ramadori G, Saile B. Portal tract fibrogenesis in the liver. Lab Invest. 2004;84(2):153–159. - PubMed
-
- Paku S, Nagy P, Kopper L, et al. 2-acetylaminofluorene dose-dependent differentiation of rat oval cells into hepatocytes: confocal and electron microscopic studies. Hepatology. 2004;39(5):1353–1361. - PubMed
-
- Knight B, Matthews VB, Olynyk JK, et al. Jekyll and Hyde: evolving perspectives on the function and potential of the adult liver progenitor (oval) cell. Bioessays. 2005;27(11):1192–1202. - PubMed
-
- Sell S. Comparison of liver progenitor cells in human atypical ductular reactions with those seen in experimental models of liver injury. Hepatology. 1998;27(2):317–331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

