Absolute bioavailability of cis-mirincamycin and trans-mirincamycin in healthy rhesus monkeys and ex vivo antimalarial activity against Plasmodium falciparum
- PMID: 21947400
- PMCID: PMC3232806
- DOI: 10.1128/AAC.01619-10
Absolute bioavailability of cis-mirincamycin and trans-mirincamycin in healthy rhesus monkeys and ex vivo antimalarial activity against Plasmodium falciparum
Abstract
The pharmacokinetics, oral bioavailability, and ex vivo antimalarial activity of mirincamycin isomers in a healthy rhesus monkey model were assessed to support lead optimization of novel nonhemolytic drugs for radical cure and causal prophylaxis of malaria. Fourteen male rhesus monkeys were randomized to four groups, which included cis and trans isomers by the oral and intravenous routes, with vehicle-only controls for each dosing route. Concentration-time data were collected for 7 days and were analyzed by noncompartmental analysis. cis-Mirincamycin had an absolute oral bioavailability of 13.6%, which was slightly higher than that of trans-mirincamycin (11.7%), but this difference was not statistically significant. There was a statistically significant difference between the area under the concentration-time curve from zero to 48 h (AUC(0-48)) of cis-mirincamycin and that of trans-mirincamycin after oral dosing. When cultured in vitro with the W2 clone of Plasmodium falciparum, the 50% inhibitory concentrations for cis-mirincamycin, trans-mirincamycin, and dihydroartemisinin were 11,300, 12,300, and 2.30 nM, respectively. However, when dosed primate plasma was cultured ex vivo against the W2 clone, both isomers had much greater relative potencies than their in vitro activities relative to results for dihydroartemisinin, an increase of approximately 100-fold for the cis isomer and 150-fold for the trans isomer. Further, oral ex vivo activity was significantly higher than intravenous activity for both isomers, particularly during the first 90 min following dosing, suggesting the first-pass formation of one or more metabolites with blood-stage antimalarial activity. Identification of the metabolic pathways and metabolites may help to further delineate the properties of this class of drugs with previously demonstrated liver-stage antimalarial activity.
Figures

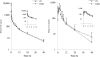

Similar articles
-
Anti-relapse activity of mirincamycin in the Plasmodium cynomolgi sporozoite-infected Rhesus monkey model.Malar J. 2014 Oct 17;13:409. doi: 10.1186/1475-2875-13-409. Malar J. 2014. PMID: 25326032 Free PMC article.
-
Mirincamycin, an old candidate for malaria combination treatment and prophylaxis in the 21st century: in vitro interaction profiles with potential partner drugs in continuous culture and field isolates.Malar J. 2014 Jun 10;13:228. doi: 10.1186/1475-2875-13-228. Malar J. 2014. PMID: 24916383 Free PMC article.
-
In vitro activity of mirincamycin (U24729A) against Plasmodium falciparum isolates from Gabon.Antimicrob Agents Chemother. 2010 Jan;54(1):540-2. doi: 10.1128/AAC.01090-09. Epub 2009 Oct 19. Antimicrob Agents Chemother. 2010. PMID: 19841147 Free PMC article.
-
Quest for a potent antimalarial drug lead: Synthesis and evaluation of 6,7-dimethoxyquinazoline-2,4-diamines.Bioorg Med Chem. 2021 Mar 1;33:116018. doi: 10.1016/j.bmc.2021.116018. Epub 2021 Jan 16. Bioorg Med Chem. 2021. PMID: 33524940 Review.
-
Antimalarial application of quinones: A recent update.Eur J Med Chem. 2021 Jan 15;210:113084. doi: 10.1016/j.ejmech.2020.113084. Epub 2020 Dec 5. Eur J Med Chem. 2021. PMID: 33333397 Review.
Cited by
-
Anti-relapse activity of mirincamycin in the Plasmodium cynomolgi sporozoite-infected Rhesus monkey model.Malar J. 2014 Oct 17;13:409. doi: 10.1186/1475-2875-13-409. Malar J. 2014. PMID: 25326032 Free PMC article.
-
Antibiotics in malaria therapy: which antibiotics except tetracyclines and macrolides may be used against malaria?Malar J. 2016 Nov 15;15(1):556. doi: 10.1186/s12936-016-1613-y. Malar J. 2016. PMID: 27846898 Free PMC article. Review.
-
Mirincamycin, an old candidate for malaria combination treatment and prophylaxis in the 21st century: in vitro interaction profiles with potential partner drugs in continuous culture and field isolates.Malar J. 2014 Jun 10;13:228. doi: 10.1186/1475-2875-13-228. Malar J. 2014. PMID: 24916383 Free PMC article.
References
-
- Fortman J., Hewett T., Bennett B. 2001. The laboratory nonhuman primate, p 17–19 CRC Press, Boca Raton, FL
-
- Fracisco S., et al. 2009. Mirincamycin: reassessment of a promising antimalarial agent with potential in a P. cynomolgi relapsing malaria monkey model, abstr. ASTMH09-298. Abstr. 58th Annu. Meet. Am. Soc. Trop. Med. Hygiene.
-
- Fracisco S., Gettayacamin Y. R. M., Westerman R., Ohrt C. 2008. Anti-malarial activity of mirincamycin and its analogs in vitro and in an in vivo presumptive causal prophylactic mouse model, abstr. ASTMH08-758. Abstr. 57th Annu. Meet. Am. Soc. Trop. Med. Hygiene.
-
- Lacy C., Armstrong L., Goldman M., Lance L. 2008. Drug information handbook, 17th ed., p. 354–357 Lexi-Comp Information Management System, Hudson, OH
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

