Beyond the intention-to-treat in comparative effectiveness research
- PMID: 21948059
- PMCID: PMC3731071
- DOI: 10.1177/1740774511420743
Beyond the intention-to-treat in comparative effectiveness research
Abstract
Background: The intention-to-treat comparison is the primary, if not the only, analytic approach of many randomized clinical trials.
Purpose: To review the shortcomings of intention-to-treat analyses, and of 'as treated' and 'per protocol' analyses as commonly implemented, with an emphasis on problems that are especially relevant for comparative effectiveness research.
Methods and results: In placebo-controlled randomized clinical trials, intention-to-treat analyses underestimate the treatment effect and are therefore nonconservative for both safety trials and noninferiority trials. In randomized clinical trials with an active comparator, intention-to-treat estimates can overestimate a treatment's effect in the presence of differential adherence. In either case, there is no guarantee that an intention-to-treat analysis estimates the clinical effectiveness of treatment. Inverse probability weighting, g-estimation, and instrumental variable estimation can reduce the bias introduced by nonadherence and loss to follow-up in 'as treated' and 'per protocol' analyses.
Limitations: These analyse require untestable assumptions, a dose-response model, and time-varying data on confounders and adherence.
Conclusions: We recommend that all randomized clinical trials with substantial lack of adherence or loss to follow-up are analyzed using different methods. These include an intention-to-treat analysis to estimate the effect of assigned treatment and 'as treated' and 'per protocol' analyses to estimate the effect of treatment after appropriate adjustment via inverse probability weighting or g-estimation.
Figures
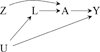
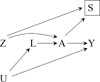
References
-
- Luce BR, Kramer JM, Goodman SN, Connor JT, Tunis S, Whicher D, Schwartz JS. Rethinking randomized clinical trials for comparative effectiveness research: the need for transformational change. Ann Intern Med. 2009;151:206–209. - PubMed
-
- Food and Drug Administration. International Conference on Harmonisation; Guidance on Statistical Principles for Clinical Trials. Federal Register. 1998;63:49583–49598. - PubMed
-
- Rosenberger WF, Lachin JM. Randomization in Clinical Trials: Theory and Practice. New York, NY: Wiley-Interscience; 2002.
-
- Piantadosi S. Clinical Trials: A Methodologic Perspective. 2nd edition. Hoboken, NY: Wiley-Interscience; 2005.
-
- Sheiner LB, Rubin DB. Intention-to-treat analysis and the goals of clinical trials. Clin Pharmacol Ther. 1995;57:6–15. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

