Comparison of printed glycan array, suspension array and ELISA in the detection of human anti-glycan antibodies
- PMID: 21948103
- PMCID: PMC3228963
- DOI: 10.1007/s10719-011-9349-y
Comparison of printed glycan array, suspension array and ELISA in the detection of human anti-glycan antibodies
Abstract
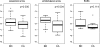
Anti-glycan antibodies represent a vast and yet insufficiently investigated subpopulation of naturally occurring and adaptive antibodies in humans. Recently, a variety of glycan-based microarrays emerged, allowing high-throughput profiling of a large repertoire of antibodies. As there are no direct approaches for comparison and evaluation of multi-glycan assays we compared three glycan-based immunoassays, namely printed glycan array (PGA), fluorescent microsphere-based suspension array (SA) and ELISA for their efficacy and selectivity in profiling anti-glycan antibodies in a cohort of 48 patients with and without ovarian cancer. The ABO blood group glycan antigens were selected as well recognized ligands for sensitivity and specificity assessments. As another ligand we selected P(1), a member of the P blood group system recently identified by PGA as a potential ovarian cancer biomarker. All three glyco-immunoassays reflected the known ABO blood groups with high performance. In contrast, anti-P(1) antibody binding profiles displayed much lower concordance. Whilst anti-P(1) antibody levels between benign controls and ovarian cancer patients were significantly discriminated using PGA (p=0.004), we got only similar results using SA (p=0.03) but not for ELISA. Our findings demonstrate that whilst assays were largely positively correlated, each presents unique characteristic features and should be validated by an independent patient cohort rather than another array technique. The variety between methods presumably reflects the differences in glycan presentation and the antigen/antibody ratio, assay conditions and detection technique. This indicates that the glycan-antibody interaction of interest has to guide the assay selection.
© The Author(s) 2011. This article is published with open access at Springerlink.com
Figures
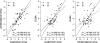

Similar articles
-
Blood Plasma-Derived Anti-Glycan Antibodies to Sialylated and Sulfated Glycans Identify Ovarian Cancer Patients.PLoS One. 2016 Oct 20;11(10):e0164230. doi: 10.1371/journal.pone.0164230. eCollection 2016. PLoS One. 2016. PMID: 27764122 Free PMC article.
-
Multiplex suspension array for human anti-carbohydrate antibody profiling.Analyst. 2011 Feb 7;136(3):560-9. doi: 10.1039/c0an00758g. Epub 2010 Nov 25. Analyst. 2011. PMID: 21107457
-
Naturally occurring anti-glycan antibodies binding to Globo H-expressing cells identify ovarian cancer patients.J Ovarian Res. 2017 Feb 10;10(1):8. doi: 10.1186/s13048-017-0305-8. J Ovarian Res. 2017. PMID: 28187738 Free PMC article.
-
Glycan-specific antibodies as potential cancer biomarkers: a focus on microarray applications.Clin Chem Lab Med. 2020 Sep 25;58(10):1611-1622. doi: 10.1515/cclm-2019-1161. Clin Chem Lab Med. 2020. PMID: 32324152 Review.
-
Natural Antibodies Against Sialoglycans.Top Curr Chem. 2015;366:169-81. doi: 10.1007/128_2013_469. Top Curr Chem. 2015. PMID: 24037491 Review.
Cited by
-
Exploration of sialic acid diversity and biology using sialoglycan microarrays.Biopolymers. 2013 Oct;99(10):650-65. doi: 10.1002/bip.22314. Biopolymers. 2013. PMID: 23765393 Free PMC article. Review.
-
Ultra-Fast Glyco-Coating of Non-Biological Surfaces.Int J Mol Sci. 2016 Jan 16;17(1):118. doi: 10.3390/ijms17010118. Int J Mol Sci. 2016. PMID: 26784187 Free PMC article.
-
Blood Plasma-Derived Anti-Glycan Antibodies to Sialylated and Sulfated Glycans Identify Ovarian Cancer Patients.PLoS One. 2016 Oct 20;11(10):e0164230. doi: 10.1371/journal.pone.0164230. eCollection 2016. PLoS One. 2016. PMID: 27764122 Free PMC article.
-
Production of a recombinant antibody specific for i blood group antigen, a mesenchymal stem cell marker.Biores Open Access. 2013 Oct;2(5):336-45. doi: 10.1089/biores.2013.0026. Biores Open Access. 2013. PMID: 24083089 Free PMC article.
-
Serum antibody screening using glycan arrays.Chem Soc Rev. 2024 Mar 4;53(5):2603-2642. doi: 10.1039/d3cs00693j. Chem Soc Rev. 2024. PMID: 38305761 Free PMC article. Review.
References
-
- Dotan I, Fishman S, Dgani Y, Schwartz M, Karban A, Lerner A, Weishauss O, Spector L, Shtevi A, Altstock RT, Dotan N, Halpern Z. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn’s disease. Gastroenterology. 2006;131(2):366–378. doi: 10.1053/j.gastro.2006.04.030. - DOI - PubMed
-
- Brettschneider J, Jaskowski TD, Tumani H, Abdul S, Husebye D, Seraj H, Hill HR, Fire E, Spector L, Yarden J, Dotan N, Rose JW. Serum anti-GAGA4 IgM antibodies differentiate relapsing remitting and secondary progressive multiple sclerosis from primary progressive multiple sclerosis and other neurological diseases. J. Neuroimmunol. 2009;217(1–2):95–101. doi: 10.1016/j.jneuroim.2009.07.017. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

